Abstract
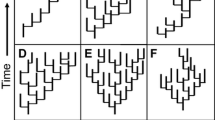
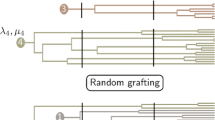
Testing whether a certain biological trait significantly affects clade diversification is central to macroevolutionary research. To this end, many scientists use constant-rate estimators (CR estimators) of diversification. However, it has never been examined whether these estimators report meaningful relationships between traits and diversification even when the diversification itself decelerates over time. In this study, I simulate trait-driven diversification concurrently with diversification slowdowns. Then, I test whether CR estimators manage to uncover the simulated relationships. Results suggest that CR estimators are robust against violation of rate constancy and successfully detect trait-dependent diversification in spite of diversification declines. Interestingly, correct results were recovered whether clade age correlated with clade diversity or not. Further comparison of CR estimators with QuaSSE suggested that QuaSSE performs better under constant diversification, but tends to report spuriously significant outcomes when diversification decelerates (=elevated Type I error). Given that diversification slowdowns have been recently reported for a wide range of taxa, these findings may be of particular relevance for future diversification studies.




Similar content being viewed by others
References
Alfaro, M. E., Santini, F., Brock, C., Alamillo, H., Dornburg, A., Rabosky, D. L., et al. (2009). Nine exceptional radiations plus high turnover explain species diversity in jawed vertebrates. Proceedings of the National Academy of Sciences of the United States of America, 106, 13410–13414.
Alroy, J. (2000). New methods for quantifying macroevolutionary patterns and processes. Paleobiology, 26, 707–733.
Blomberg, S. P., Garland, T., & Ives, A. R. (2003). Testing for phylogenetic signal in comparative data: Behavioral traits are more labile. Evolution, 57, 717–745.
Blum, M. G. B., & Francois, O. (2006). Which random processes describe the tree of life? A large-scale study of phylogenetic tree imbalance. Systematic Biology, 55, 685–691.
Bokma, F. (2008a). Bayesian estimation of speciation and extinction probabilities from (in)complete phylogenies. Evolution, 62, 2441–2445.
Bokma, F. (2008b). Detection of punctuated equilibrium by Bayesian estimation of speciation and extinction rates, ancestral character states, and rates of anagenetic and cladogenetic evolution on a molecular phylogeny. Evolution, 62, 2718–2726.
Bortolussi, N., Durand, E., Blum, M., & Francois, O. (2006). apTreeshape: Statistical analysis of phylogenetic tree shape. Bioinformatics, 22, 363–364.
Butler, M. A., & King, A. A. (2004). Phylogenetic comparative analysis: A modeling approach for adaptive evolution. American Naturalist, 164, 683–695.
Davies, T. J., Barraclough, T. G., Chase, M. W., Soltis, P. S., Soltis, D. E., & Savolainen, V. (2004). Darwin’s abominable mystery: Insights from a supertree of the angiosperms. Proceedings of the National Academy of Sciences of the United States of America, 101, 1904–1909.
Etienne, R. S., Haegeman, B., Stadler, T., Aze, T., Pearson, P. N., Purvis, A., et al. (2012). Diversity-dependence brings molecular phylogenies closer to agreement with the fossil record. Proceedings of the Royal Society B Biological Sciences, 279, 1300–1309.
Felsenstein, J. (1985). Phylogenies and the comparative method. American Naturalist, 125, 1–15.
FitzJohn, R. G. (2010). Quantitative traits and diversification. Systematic Biology, 59, 619–633.
FitzJohn, R. G. (2012). Diversitree: Comparative phylogenetic analyses of diversification in R. Methods in Ecology and Evolution, 3, 1084–1092.
Foote, M. (2000). Origination and extinction components of taxonomic diversity: General problems. Paleobiology, 26, 74–102.
Freckleton, R. P., Harvey, P. H., & Pagel, M. (2002). Phylogenetic analysis and comparative data: A test and review of evidence. American Naturalist, 160, 712–726.
Gittleman, J. L., & Kot, M. (1990). Adaptation—statistics and a null model for estimating phylogenetic effects. Systematic Zoology, 39, 227–241.
Glor, R. E. (2010). Phylogenetic insights on adaptive radiation. Annual Review of Ecology Evolution and Systematics, 41, 251–270.
Good-Avila, S. V., Souza, V., Gaut, B. S., & Eguiarte, L. E. (2006). Timing and rate of speciation in Agave (Agavaceae). Proceedings of the National Academy of Sciences of the United States of America, 103, 9124–9129.
Harmon, L. J., Weir, J. T., Brock, C. D., Glor, R. E., & Challenger, W. (2008). GEIGER: Investigating evolutionary radiations. Bioinformatics, 24, 129–131.
Hohna, S., Stadler, T., Ronquist, F., & Britton, T. (2011). Inferring speciation and extinction rates under different sampling schemes. Molecular Biology and Evolution, 28, 2577–2589.
Hunt, T., Bergsten, J., Levkanicova, Z., Papadopoulou, A., John, O. S., Wild, R., et al. (2007). A comprehensive phylogeny of beetles reveals the evolutionary origins of a superradiation. Science, 318, 1913–1916.
Hutchinson, G. E. (1959). Homage to Santa Rosalia or why are there so many kinds of animals. American Naturalist, 93, 145–159.
Ingram, T. (2011). Speciation along a depth gradient in a marine adaptive radiation. Proceedings of the Royal Society B Biological Sciences, 278, 613–618.
Jablonski, D. (2008). Species selection: Theory and data. Annual Review of Ecology Evolution and Systematics, 39, 501–524.
Jansson, R., & Davies, T. J. (2008). Global variation in diversification rates of flowering plants: Energy vs. climate change. Ecology Letters, 11, 173–183.
Klak, C., Reeves, G., & Hedderson, T. (2004). Unmatched tempo of evolution in Southern African semi-desert ice plants. Nature, 427, 63–65.
Maddison, W. P. (2006). Confounding asymmetries in evolutionary diversification and character change. Evolution, 60, 1743–1746.
Maddison, W. P., Midford, P. E., & Otto, S. P. (2007). Estimating a binary character’s effect on speciation and extinction. Systematic Biology, 56, 701–710.
Magallon, S., & Sanderson, M. J. (2001). Absolute diversification rates in angiosperm clades. Evolution, 55, 1762–1780.
McPeek, M. A. (2008). The ecological dynamics of clade diversification and community assembly. American Naturalist, 172, 270–284.
Mittelbach, G. G., Schemske, D. W., Cornell, H. V., Allen, A. P., Brown, J. M., Bush, M. B., et al. (2007). Evolution and the latitudinal diversity gradient: Speciation, extinction and biogeography. Ecology Letters, 10, 315–331.
Moore, B. R., & Donoghue, M. J. (2009). A Bayesian approach for evaluating the impact of historical events on rates of diversification. Proceedings of the National Academy of Sciences of the United States of America, 106, 4307–4312.
Morlon, H., Potts, M. D., & Plotkin, J. B. (2010). Inferring the dynamics of diversification: A coalescent approach. PLoS Biology, 89, e1000493.
Nee, S. (2006). Birth-death models in macroevolution. Annual Review of Ecology Evolution and Systematics, 2006(37), 1–17.
Nee, S., May, R. M., & Harvey, P. H. (1994). The reconstructed evolutionary process. Philosophical Transactions of the Royal Society B Biological Sciences, 344, 305–311.
Nylin, S., & Wahlberg, N. (2008). Does plasticity drive speciation? Host-plant shifts and diversification in nymphaline butterflies (Lepidoptera: Nymphalidae) during the tertiary. Biological Journal of the Linnean Society, 94, 115–130.
Paradis, E. (2004). Can extinction rates be estimated without fossils? Journal of Theoretical Biology, 229, 19–30.
Paradis, E. (2005). Statistical analysis of diversification with species traits. Evolution, 59, 1–12.
Paradis, E. (2008). Asymmetries in phylogenetic diversification and character change can be untangled. Evolution, 62, 241–247.
Paradis, E., Claude, J., & Strimmer, K. (2004). APE: Analyses of phylogenetics and evolution in R language. Bioinformatics, 20, 289–290.
Pennell, M. W., Sarver, B. A. J., & Harmon, L. J. (2012). Trees of unusual size: Biased inference of early bursts from large molecular phylogenies. PLoS ONE, 7, e43348.
Phillimore, A. B., & Price, T. D. (2008). Density-dependent cladogenesis in birds. PLoS Biology, 6, e0071.
Pybus, O. G., & Harvey, P. H. (2000). Testing macro-evolutionary models using incomplete molecular phylogenies. Proceedings of the Royal Society B Biological Sciences, 267, 2267–2272.
R Development Core Team. (2012). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing.
Rabosky, D. L. (2006). Likelihood methods for detecting temporal shifts in diversification rates. Evolution, 60, 1152–1164.
Rabosky, D. L. (2009a). Ecological limits and diversification rate: Alternative paradigms to explain the variation in species richness among clades and regions. Ecology Letters, 12, 735–743.
Rabosky, D. L. (2009b). Ecological limits on clade diversification in higher taxa. American Naturalist, 173, 662–674.
Rabosky, D. L. (2010a). Extinction rates should not be estimated from molecular phylogenies. Evolution, 64, 1816–1824.
Rabosky, D. L. (2010b). Primary controls on species richness in higher taxa. Systematic Biology, 59, 634–645.
Rabosky, D. L., & Adams, D. C. (2012). Rates of morphological evolution are correlated with species richness in salamanders. Evolution, 66, 1807–1818.
Rabosky, D. L., & Lovette, I. J. (2008). Density-dependent diversification in North American wood warblers. Proceedings of the Royal Society B Biological Sciences, 275, 2363–2371.
Rabosky, D. L., Slater, G. J., & Alfaro, M. E. (2012). Clade age and species richness are decoupled across the eukaryotic tree of life. PLoS Biology, 10, e1001381.
Raup, D. M. (1985). Mathematical models of cladogenesis. Paleobiology, 11, 42–52.
Ricklefs, R. E. (2006). Global variation in the diversification rate of passerine birds. Ecology, 87, 2468–2478.
Ricklefs, R. E. (2007). Estimating diversification rates from phylogenetic information. Trends in Ecology & Evolution, 22, 601–610.
Sanderson, M. J., & Donoghue, M. J. (1996). Reconstructing shifts in diversification rates on phylogenetic trees. Trends in Ecology & Evolution, 11, 15–20.
Schluter, D., Price, T., Mooers, A. O., & Ludwig, D. (1997). Likelihood of ancestor states in adaptive radiation. Evolution, 51, 1699–1711.
Schnitzler, J., Barraclough, T. G., Boatwright, J. S., Goldblatt, P., Manning, J. C., Powell, M. P., et al. (2011). Causes of plant diversification in the cape biodiversity hotspot of South Africa. Systematic Biology, 60, 343–357.
Sepkoski, J. J. (1998). Rates of speciation in the fossil record. Philosophical Transactions of the Royal Society B Biological Sciences, 353, 315–326.
Silvestro, D., Schnitzler, J., & Zizka, G. (2011). A Bayesian framework to estimate diversification rates and their variation through time and space. BMC Evolutionary Biology, 11, 311.
Stadler, T. (2009). On incomplete sampling under birth-death models and connections to the sampling-based coalescent. Journal of Theoretical Biology, 261, 58–66.
Stadler, T. (2011). Mammalian phylogeny reveals recent diversification rate shifts. Proceedings of the National Academy of Sciences of the United States of America, 108, 6187–6192.
Vamosi, J. C., & Vamosi, S. M. (2010). Key innovations within a geographical context in flowering plants: Towards resolving Darwin’s abominable mystery. Ecology Letters, 13, 1270–1279.
Wertheim, J. O., & Sanderson, M. J. (2011). Estimating diversification rates: How useful are divergence times? Evolution, 65, 309–320.
Weston, S. J. (2013). FOREACH: R package for parallel computing. Palo Alto, USA: Revolutionary Analytics.
Wiens, J. J. (2011). The causes of species richness patterns across space, time, and clades and the role of ecological limits. The Quarterly Review of Biology, 86, 75–96.
Wiens, J. J., Graham, C. H., Moen, D. S., Smith, S. A., & Reeder, T. W. (2006). Evolutionary and ecological causes of the latitudinal diversity gradient in hylid frogs: Treefrog trees unearth the roots of high tropical diversity. American Naturalist, 168, 579–596.
Acknowledgments
I am thankful to Luke Harmon, Dan Rabosky, John Wiens and members of his lab for insightful comments on earlier versions of the manuscript. They may not necessarily endorse everything I have written. Computational capacity was lent by the NGI MetaCentrum, provided under the program “Projects of large infrastructure for research, development, and innovations” (LM2010005). This work was supported by the Grant Agency of the Czech Republic (P505/11/2387) and the J. W. Fulbright Commission.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Machac, A. Detecting Trait-Dependent Diversification Under Diversification Slowdowns. Evol Biol 41, 201–211 (2014). https://doi.org/10.1007/s11692-013-9258-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11692-013-9258-z