Abstract
Microstructural alterations in white matter are evident in obsessive–compulsive disorder (OCD) both in adult and paediatric populations. Paediatric patients go through the process of maturation and thus may undergo different pathophysiology than adult OCD. Findings from studies in paediatric obsessive–compulsive disorder have been inconsistent, possibly due to their small sample size or heterogeneous populations. The aim of this review is to provide a comprehensive overview of white matter structures in paediatric obsessive–compulsive disorder and their correlation with clinical features. Based on PRISMA guidelines, we performed a systematic search on diffusion tensor imaging studies that reported fractional anisotropy, mean diffusivity, radial diffusivity, or axial diffusivity alterations between paediatric patients with obsessive–compulsive disorder and healthy controls using voxel-based analysis, or tract‐based spatial statistics. We identified fifteen relevant studies. Most studies reported changes predominantly in the corpus callosum, cingulum, arcuate fasciculus, uncinate fasciculus, inferior longitudinal fasciculus, superior longitudinal fasciculus, inferior fronto-occipital fasciculus, corticospinal tract, forceps minor and major, and the cerebellum in paediatric obsessive–compulsive disorder. These alterations included increased and decreased fractional anisotropy and radial diffusivity, and increased mean and axial diffusivity in different white matter tracts. These changes were associated with obsessive–compulsive disorder symptoms. Moreover, specific genetic polymorphisms were linked with cerebellar white matter changes in paediatric obsessive–compulsive disorder. White matter changes are widespread in paediatric OCD patients. These changes are often associated with symptoms however there are controversies in the direction of changes in some tracts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obsessive–compulsive disorder (OCD) is the fourth most common mental disorder with a lifetime prevalence of approximately 2–3%. OCD is characterized by recurrent obsessive thoughts and intrusions (obsessions) and habitual behaviours (compulsions). These symptoms disturb patients’ daily activity and affect their quality of life (Drubach, 2015; Ferreira et al., 2020). Around 1–3% of children experience OCD symptoms and unlike adult OCD, boys are more commonly affected by the symptoms than girls. The mean age of paediatric OCD is ten years but symptoms may appear in children as young as five years old (Mataix-Cols et al., 2008).
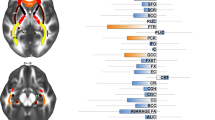
The aetiology of OCD has been linked to various brain systems. Earlier hypotheses were based on clinical observations from pallidal and frontal lobe lesions (Eslinger & Damasio, 1985) but data obtained from brain imaging in the last three decades revolutionized our knowledge of underlying neurobiology. The current models are focused on the orbitofronto-striatal circuit (Stein, 2002). This circuit includes medial orbitofrontal, anterior cingulate and temporolimbic cortices, striatum, and thalamus. The anterior cingulate cortex, the dorsolateral prefrontal cortex, and the orbitofrontal cortex seem to be more relevant to the psychopathology of OCD (Chamberlain et al., 2008) (Fig. 1). However, recent neuroimaging studies in OCD suggest that this model may not be sufficient to explain the diverse clinical manifestations of OCD. A broader model highlights involvements of other structures such as dorsolateral prefronto-striatal circuit (dorsomedial, dorsolateral, ventrolateral, and frontopolar prefrontal cortices), and reciprocally connected temporo-parieto-occipital associative areas. The notion indicates network pathology rather than a specific anatomical one. Alternatively, OCD may be a heterogenous condition with various neural pathology but common psychiatric symptoms (Menzies et al., 2008a, b; Piras et al., 2015).
Neural circuits involved in OCD. Abbreviations: SMA=Supplementary motor area, DlPFC=dorsolateral prefrontal cortex, DmPFC=Dorsomedial prefrontal cortex, IFG=Inferior frontal gyrus, vlPFC=Ventromedial prefrontal cortex, PFC=Orbitofrontal cortex, vmPFC=Ventromedial prefrontal cortex, dPut=dorsal putamen, dCaud=Dorsal caudate, vCaud=Ventral caudate, NAcc=Nucleus accumbens
Diffusion tensor imaging and white matter alterations
Diffusion tensor imaging (DTI) allows the assessment of WM integrity within the brain major tracts. The method has been applied to study white matter changes in the OCD (Correia et al., 2008; Piras et al., 2021a, b, c). DTI has been widely used in neurodevelopmental (Abdolalizadeh et al., 2021; Ghazi Sherbaf et al., 2019; Piras et al., 2021a, b, c; Piras et al., 2013) and neurocognitive studies too (Seyedmirzaei et al., 2022).
The principle of DTI is the measurement of diffusion of water in the brain tissue using changes in the radiofrequency signal that occurs as water molecules move towards or away from the source of radiofrequency. Water randomly diffuses in all directions in an unbounded environment. However, in the WM the direction of water diffusivity is mainly aligned with neural pathways as axonal membranes and myelin limit its radial diffusivity.
There are four main parameters often used as surrogate markers of diffusion in DTI studies. These markers indirectly represent microstructure of neural fibers in the brain. Fractional anisotropy (FA) is the most common indicator among these and represents the degree of anisotropy, which in turn is computed from the eigenvalues of the diffusion tensors in each of the axes. FA is particularly affected by density, orientation, WM integrity and myelination in each voxel. It decreases in neural pathology that affects myelination and orientation of the fibres. The highest FA values are found in corpus callosum and internal capsule, and the lowest values are in the grey matter and in voxels containing crossing fibers (DeBoy et al., 2007). The FA values in dense and well-aligned WM tracts are higher, whereas the FA values in CSF and damaged fibers are lower (Smith et al., 2006). Determination of FA and calculation of eigenvectors are the principle upon which DT tractography is modelled. Axial diffusivity (AD) refers to the diffusion along the main axis (principal eigenvector) of the diffusion model, and radial diffusivity (RD) refers to the diffusion vector perpendicular to the main eigenvector, which is calculated as the mean of secondary and tertiary eigenvectors (Song et al., 2003). Studies in mice showed that AD reflects axonal damage, whereas RD is affected by myelination (Frydman et al., 2016; Song et al., 2003). These two parameters particularly change with aging (Bennett et al., 2010). Mean diffusivity (MD) is the average magnitude of diffusivity along the xyz directions. MD is a non-specific, but sensitive, metric and is influenced by any condition which restricts diffusion of water freely (Bosch et al., 2012). DTI resolution does not allow imaging at the axonal level but provides information that is relevant to neural tract anatomy usually at a 2–3 mm3 scale.
There are different approaches to the analysis of DTI data in order to assess WM microstructure. Voxel-based morphometry (VBM) is probably the most common approach. In this method, diffusion data is aligned with a high-resolution template, which is spatially normalized and smoothened prior to running statistical tests. As a result, the outcomes are highly reliant on a variety of parameters, such as the accuracy of the registration, smoothing filter size, etc. (Jones et al., 2005). Tract-based spatial statistics (TBSS) is another approach (Smith et al., 2006) that is more robust than VBM. In this method, each individual's FA data is projected onto a skeleton, which is constructed from the center of major white matter tracts. This will substantially remove the potential errors, which may arise from registration and partial volume effect. The analysis of DTI data by region of interest (ROI) is another frequently utilized approach. In this approach, regions are defined using an atlas, or by automatic or manual segmentation of the ROI. Various DTI measures can then be calculated. Structural connectivity between different brain regions can be characterized non-invasively and in vivo, by using a fibre-tracking algorithm. The stepwise production of streamlines is the foundation of the most used fibre-tracking technique. Using this technique, it is not possible to differentiate between some scenarios solely at the voxel level since varied local fibre geometries, such as crossing, kissing, bending, and fanning, might result in the same MRI data (Jeurissen et al., 2019).
Neural basis of OCD
Neuroimaging findings in OCD strongly suggest the involvement of “affective” fronto-striatal loop, which comprises of orbitofrontal cortex, anterior cingulate, striatum, thalamus, and temporolimbic regions (Menzies et al., 2008a, b). Functional studies suggest dysfunction in the network responsible for action selection based on the associated reward, which again involves orbitofrontal cortex, anterior cingulate cortex, rostral cingulate motor area, and motor cortex (Menzies et al., 2008a, b). “Executive” dorsolateral prefronto-striatal circuit, is also involved in OCD. This circuit includes frontal regions like dorsolateral, ventrolateral, and frontopolar prefrontal cortex and more posterior parts of the brain like temporal, parietal, and occipital lobes. Adjacent WM alterations were observed in these regions (Menzies et al., 2008a, b; Piras et al., 2015).
In a large study of cortical morphometry in OCD (Boedhoe et al., 2018) authors found that the surface area for the transverse temporal cortex was significantly decreased in adult OCD compared to healthy controls. They also had a significantly thinner inferior parietal cortex. Medicated adult patients also displayed thinner cortices across the brain. In comparison, paediatric OCD patients had significantly thinner superior and inferior parietal cortices, and medicated OCD patients showed the lower surface area in frontal regions (Boedhoe et al., 2018).
Other meta-analyses and mega-analyses of VBM studies of OCD, showed a smaller volume of the dorsomedial prefrontal cortex, dorsal anterior cingulate cortex, and bilateral insula-operculum and a greater volume of the thalamus, cerebellum, and ventral part of the putamen (Christian et al., 2008; Van den Heuvel et al., 2022). The striatal finding, in particular, is associated with the disease duration in OCD (Weeland et al., 2022). OCD-related thalamic volume differences are driven by both age and medication status (Boedhoe et al., 2018; Van den Heuvel et al., 2022; Zarei et al., 2011).
A study by Togao and colleagues (Togao et al., 2010) designed to assess morphometry in adult OCD compared to healthy controls demonstrated that the right premotor area, right orbitofrontal cortex, right dorsolateral prefrontal cortex, bilateral temporal and occipital areas had decreased grey matter volume. Additionally, they discovered a substantial decrease in WM volume in the left anterior cingulate gyrus and a significant increase in WM volume in the right orbitofrontal region and right anterior limb of the internal capsule (Togao et al., 2010).
DTI studies of adult OCD found reduced FA in the genu and splenium of the corpus callosum, cingulum bundle, superior longitudinal fasciculus, corona radiata, and orbitofrontal WM and increased FA in connections related to amygdala and parieto-occipital area (Hu et al., 2020; Piras et al., 2021a, b, c, 2013). Additionally, patients with adult OCD showed thinner corpus callosum compared to controls and to patients with other mental disorders (Piras et al., 2021c). Higher RD in the genu and body of the corpus callosum was reported in the adult OCD (Gan et al., 2017; Magioncalda et al., 2016; Rus et al., 2017; Zhou et al., 2018). Koch et al. reviewed DTI studies on adult and paediatric OCD patients and found that WM FA is often reduced in cingulum bundle, corpus callosum, and anterior limb of the internal capsule in adult OCD. However, FA and WM connectivity were increased in paediatric OCD (Koch et al., 2014). The ENIGMA consortium (Piras et al., 2021a, b, c), conducted the largest meta-analysis of WM in OCD. They found significant FA changes in the sagittal stratum, and posterior thalamic radiation in adult OCD compared to healthy controls. Further changes including higher MD in sagittal stratum and higher RD in posterior thalamic radiation and sagittal stratum were specific to adult OCD. They did not find any detectable WM changes in paediatric OCD.
Clinical parameters known to have a meaningful association with WM microstructure changes differ between paediatric and adult patients (such as disease duration or long-term pharmaceutical therapy) (Ashraf-Ganjouei et al., 2019; Benedetti et al., 2013). These patient groups also differ from each other depending on the stage of development of white matter or myelination. During childhood and adolescence, WM anisotropy changes in brain regions responsible for attention, cognition, and motor ability. Studies showed that with aging, FA values increased in the corpus callosum, arcuate fasciculus, prefrontal regions, basal ganglia, internal capsule, thalamic pathways, and ventral visual pathways (Barnea-Goraly et al., 2005; Giorgio et al., 2010).
Paediatric OCD can be profoundly different from adult cases due to the process of brain maturation, clinical symptomatology, disease duration and effect of medication use. In this study, we aimed to systematically review DTI studies in paediatric patients with OCD to provide a comprehensive overview of WM changes in this condition.
Methods and materials
Eligibility criteria
DTI studies of OCD patients under the age of 18 years were included in this study. Review articles, case reports, commentaries and letters, and animal studies were excluded.
Literature search
We performed a systematic review of the literature based on the PRISMA framework (http://www.prisma-statement.org). PubMed, EMBASE, and Scopus databases were screened till January 2022 to identify studies with the issue of DTI changes in paediatric OCD patients, applying the search term: ("Obsessive–Compulsive Disorder"[Mesh] OR "Obsessive–Compulsive Disorder" OR OCD OR "Anankastic Personality" OR "Neurosis") AND ("Diffusion Tensor Imaging"[Mesh] OR "Diffusion Tensor Imaging" OR DTI OR "diffusion MRI" OR "dMRI" OR "diffusion magnetic resonance imaging") in PubMed website. ('obsessive–compulsive disorder' OR OCD OR neurosis) AND ('Diffusion Tensor Imaging' OR DTI OR 'diffusion MRI' OR 'diffusion magnetic resonance imaging') in Embase and Scopus websites. This search was completed with no prior restrictions. Obtained results were added to the Covidence website (https://www.covidence.org). Figure 2 illustrates our process of screening and study selection based on the PRISMA guidelines.
This review was not pre-registered.
Screening and data extraction
The screening was performed by two investigators (M.H and S.P.M). First, titles and abstracts were screened, and eligible studies were chosen for full-text screening. Then, we obtained full text of the eligible articles. Finally, paediatric OCD whole brain or ROI DTI studies with full text were included in this study. After performing data extraction, we recorded the demographic and clinical profile of participants in these studies (Table 1) and used a separate table to record the imaging modalities and findings (Table 2). Table 2 demonstrates between-group discrepancy of participants with OCD compared with healthy control participants and significant correlations between diffusivity values and symptom severity in OCD patients.
Risk of bias assessment: We used the “Newcastle – Ottawa Quality Assessment Scale” (NOS) (Peterson et al., 2011) which is a widely used scale to assess the risk of bias in observational studies (or clinical trials) with ratings of biases arising from the selection, comparability, on a scale of 0–9 (Table 3).
Results
We initially found 3634 articles, of which 626 were duplicates and therefore removed. We screened a total of 3008 articles by title and abstract to decide if these studies met any of the exclusion criteria. 105 studies were selected afterward, and full-text screening yielded 15 articles that were included in this review (Fig. 2).
All of the studies included in this review were assessed for selection, comparability, and exposure based on the Newcastle–Ottawa Scale (NOS) (Peterson et al., 2011). The median score for the included studies was 9 (4–9). Two studies focused on obsessive–compulsive symptoms and only included healthy participants with no control group thus their NOS score was low (Gasso et al., 2015; Grazioplene et al., 2022). Three studies lacked one score due to poor control selection (Pagliaccio et al., 2021; Tikoo et al., 2021; Zarei et al., 2011) and the other studies reached the complete NOS score.
DTI findings in paediatric OCD patients
Studies revealed altered integrity of association fibers, including the cingulum, inferior longitudinal fasciculus, superior longitudinal fasciculus, inferior fronto-occipital fasciculus, uncinate fasciculus, and arcuate fasciculus; commissural fibers including corpus callosum, posterior limb of the internal capsule, thalamic radiation, corona radiata, and both forceps major and minor; and projection fibers including the corticospinal tract. Studies showed diverse changes in FA value in widespread regions of WM. Most studies demonstrated MD, AD, and RD values were increased in paediatric OCD patients compared to healthy controls. However, there was a report of lower RD in paediatric OCD patients compared to healthy controls in four WM areas: the left dorsal cingulum bundle, the splenium, the right corticospinal tract, and the left inferior fronto-occipital fasciculus (Gruner et al., 2012). There was a trend of FA reduction in paediatric OCD patients compared to healthy controls. However, two studies revealed higher FA in paediatric OCD patients than in HC in the left inferior longitudinal fasciculus, bilateral superior longitudinal fasciculus, right and left inferior fronto-occipital fasciculus, bilateral corticospinal tract, corpus callosum, splenium and genu, bilateral forceps major, bilateral forceps minor, left dorsal cingulum bundle and right uncinate fasciculus (Gruner et al., 2012; Zarei et al., 2011). Two studies did not show any WM tract alterations in paediatric OCD (Pagliaccio et al., 2020; Piras et al., 2021a, b, c).
The study of the ENIGMA group (Piras et al., 2021a, b, c) is the largest of its kind in terms of the number of cases included. Surprisingly, this study did not report any WM alterations in paediatric OCD. There are several methodological considerations that might have affected the result of this study. Firstly, meta-analysis although increases the power of the study by combining several smaller studies, only deals with the main effect and increases the homogeneity of the cohort, leading to the potential loss of a real effect which may be limited to a subpopulation of the cohort (Mavridis et al., 2018). For example, the rate of hoarding was almost twice as much in paediatric OCD (41%) in comparison to adult OCD (22%). Participants of smaller prospective studies are more precisely characterised, and the data is more accurately analysed. Secondly, the study used TBSS to obtain FA maps but did not perform voxel-wise comparison of FA skeletons. Group comparison was carried out using average FA value in 25 predefined tracts, extracted from an Atlas. This approached practically eliminate any chance of detecting regional changes within the tracts. Thirdly, AD, MD and RD were only assessed in the tracts that were showed to have significant group difference in FA. Fourthly, not all the studies included in the ENIGMA group study used the same method of analysis, some used TBSS and some used VBA. All these factors may potentially increase the homogeneity of the cohort leading to increased variability.
Two of the studies performed VBA analysis which could yield false positive results in the voxels close to the edge of WM (Gasso et al., 2015; Lazaro et al., 2014), and two other study used tractography technique to evaluate the structural connectivity (Grazioplene et al., 2022; Pagliaccio et al., 2020). The rest of the studies used the TBSS method. One study purely focused on cerebellar involvement (Tikoo et al., 2021).
Six studies showed that FA measures in the corpus callosum were significantly different in paediatric OCD compared to HCs. Two studies demonstrated that paediatric OCD patients had higher FA values in splenium and genu of the corpus callosum (Gruner et al., 2012; Zarei et al., 2011). FA tends to increase in the early puberty (Brouwer et al., 2012). Participants in one of our previous studies had a mean age of over sixteen years (Zarei et al., 2011). The other four studies showed that FA was significantly lower in paediatric OCD in anterior corpus callosum regions and also in splenium and genu of the corpus callosum (Ameis et al., 2016; Fitzgerald et al., 2014a, b; Lazaro et al., 2014; Rosso et al., 2014).
MD changes in the corpus callosum were prominent in merely one study; Lazaro and colleagues found increased MD in the anterior region of the corpus callosum (Lazaro et al., 2014). AD values were altered in the corpus callosum tract in three studies. These alterations were in the genu, body, splenium, and anterior parts of the corpus callosum (Jayarajan et al., 2012; Lazaro et al., 2014; Rosso et al., 2014). RD changes were manifested in the corpus callosum in four studies. These changes appeared across the corpus callosum in splenium, genu, body, or the anterior part of the corpus callosum. These parts showed higher RD in paediatric OCD in comparison with healthy controls (Gruner et al., 2012; Jayarajan et al., 2012; Lazaro et al., 2014; Rosso et al., 2014). Lazaro et al. study had the largest sample size and found changes in all diffusivity metrics in the corpus callosum (Lazaro et al., 2014). They highlighted the role of the corpus callosum in OCD neurobiology. Whole brain studies which used TBSS analysis in OCD patients found altered corpus callosum WM integrity with two exceptions (Piras, et al., 2021a, b, c; White et al., 2015). White et al. used a 1.5 T scanner; a weaker magnet that would give less detailed images. Interestingly, they were the only study to find FA reduction in the right thalamic radiation in OCD patients compared to controls and this was their only significant finding.
Based on the included studies, forceps minor and forceps major are the other commissural fibers involved in paediatric OCD. Forceps minor connects the bilateral frontal lobes which carries orbitofronto-striatal fibers, and forceps major connects the bilateral occipital lobes and is not relevant to affective or executive circuits. We previously found that both of these tracts have higher FA in paediatric OCD compared to the healthy controls (Zarei et al., 2011). This study is also unique as it found a relationship between white and grey matter changes in OCD.
Six studies showed cingulum involvement in paediatric OCD. The left dorsal cingulum bundle showed higher FA in paediatric OCD. There was also a decreased FA in the anterior cingulum bundle of paediatric OCD patients compared to healthy controls (Fitzgerald et al., 2014a, b; Gruner et al., 2012; Zarei et al., 2011). Significantly higher MD and AD and lower RD were observed in the left dorsal cingulum bundle of paediatric OCD patients compared to healthy controls (Gruner et al., 2012; Jayarajan et al., 2012; Lazaro et al., 2014). Pagliaccio et al. showed decreased streamline count in the left anterior cingulate cortex of OCD patients (Pagliaccio et al., 2020). Two studies found uncinate fasciculus DTI changes in paediatric OCD. We previously found increased FA while Jayarajan and colleagues. Reported increased RD (Jayarajan et al., 2012; Zarei et al., 2011). One study showed lower FA in the arcuate fasciculus in paediatric OCD compared to healthy controls (Ameis et al., 2016). One study showed FA increase (Zarei et al., 2011) and another one showed AD increase in paediatric OCD in the superior longitudinal fasciculus, and inferior longitudinal fasciculus (Jayarajan et al., 2012). They also found bilateral RD increases in the superior longitudinal fasciculus in paediatric OCD. Four studies found alterations in inferior-fronto-occipital fasciculus integrity. These changes included increased FA (Gruner et al., 2012; Zarei et al., 2011) and decreased FA (Ameis et al., 2016), increased (Jayarajan et al., 2012), and decreased RD (Gruner et al., 2012), and increased AD values (Gruner et al., 2012) in paediatric OCD. Among projection fibers, cerebellar peduncles and corticospinal tract were found to be involved in paediatric OCD. While corticospinal tract is a part of “Executive” dorsolateral prefronto-striatal circuit, cerebellar peduncles were not previously attributed to OCD pathophysiology (Menzies et al., 2008a, b).
Four of the studies included in this review showed corticospinal tract alterations in paediatric OCD patients. Studies by Gruner and colleagues as well as our own showed that patients with paediatric OCD displayed higher FA values compared to healthy controls in the corticospinal tract (Gruner et al., 2012; Zarei et al., 2011). However, Ameis and colleagues showed lower FA in paediatric OCD patients versus healthy controls in the corticospinal tract (Ameis et al., 2016). Pagliaccio and colleagues in a large sample study found that paediatric OCD patients had lower FA in the lower parietal superior part of the corticospinal tract compared to HC (Pagliaccio et al., 2021). There were no reports of MD or AD changes in the corticospinal tract in paediatric OCD.
Three studies showed cerebellum WM or its peduncles are involved in paediatric OCD. Lazaro and colleagues showed higher MD, AD, and RD values in the anterior and posterior lobes of the cerebellum and pons (Lazaro et al., 2014). Gasso and colleagues found higher MD in the anterior and posterior lobe of the right cerebellum and culmen and lingual lobes of the left cerebellum (Gasso et al., 2015). Tikoo and colleagues, (Tikoo et al., 2021) showed that paediatric patients with Tourette’s syndrome and OCD had higher FA in all three cerebellar peduncles but patients with mere OCD diagnosis, had lower FA in all three cerebellar peduncles compared to healthy controls. The importance of cerebellum in OCD has also been highlighted by functional MRI study that showed decreased functional connectivity of dentate nucleus with the left crus II of the cerebellum. Among all the above studies the most consistent findings were changes in the cingulum tract, inferior-fronto-occipital tract, and corticospinal tract. Most studies used TBSS analysis, except one which reported higher MD in the cingulum tract (Lazaro et al., 2014) and another which reported a lower streamline count in the left anterior cingulate cortex of OCD patients (Pagliaccio et al., 2020).
Sex-specific pattern and DTI parameters
The study by Fitzgerald et al. investigated the effects of age and sex on FA in each group of paediatric OCD patients and healthy controls in the corpus callosum, cingulum bundle, and anterior limb of the internal capsule. Their data showed that the increase of FA in patients compared with healthy controls is related to age. This relationship was much more prominent for girls in the anterior cingulum bundle. Moreover, they found lower FA in the genu of the corpus callosum in boys compared to girls (Fitzgerald et al., 2014a, b). Gasso et al. found that gender was linked with MD values in a cluster involving the inferior frontal gyrus and lentiform nucleus in OCD patients (Gasso et al., 2015).
Clinical correlations and DTI parameters
WM FA of many regions revealed a significant positive correlation with the symptoms’ severity. These regions included the left uncinate fasciculus, corticospinal tract, superior longitudinal fasciculus, forceps major, forceps minor, superior corona radiata, splenium, anterior thalamic radiation, and posterior limb of the internal capsule. We previously showed a significant negative correlation between the left hippocampal cingulum bundle adjacent to the entorhinal cortex bilaterally and the symptom severity (Zarei et al., 2011). However, Gruner et al. study did not find any significant correlations between symptom severity and FA values of the left dorsal cingulum bundle, splenium of corpus callosum, right corticospinal tract, and the left inferior fronto-occipital fasciculus. These fibers showed higher FA in patients compared to healthy controls (Gruner et al., 2012).
One study showed that total obsession score was significantly associated with higher FA in the splenium of corpus callosum. (Gruner et al., 2012) In addition they found that executive functions had a significant direct correlation with the left dorsal cingulum bundle FA. Presenting harm and checking symptoms were accompanied by decreased FA in corpus callosum, left anterior cingulate gyrus, and anterior region of the left caudate nucleus, while contamination and washing symptoms correlated with decreased FA in the left midbrain, lentiform nucleus, insula, and thalamus. Adaptive functioning scores were positively correlated with FA in the genu and splenium of the corpus callosum, corticospinal tract, inferior longitudinal fasciculus, arcuate fasciculus, and inferior fronto-occipital fasciculus (Ameis et al., 2016). Adaptive functioning scores positively correlated with FA among patients, particularly in genu and splenium of corpus callosum, as well as in the corticospinal tract, inferior longitudinal fasciculus, arcuate fasciculus, inferior fronto-occipital fasciculus (Ameis et al., 2016).
Gruner et al. observed FA alterations in the left cingulum bundle associated with total obsessions scores and executive functioning scores (Gruner et al., 2012). Larazo and colleagues’ findings were in line with the Gruner study (Lazaro et al., 2014). They found that the left anterior cingulate gyrus changes correlated with presenting harm and checking symptoms.
As measured in the Child Behaviour Checklist-Obsessive Compulsive Scale (CBCL-OCS), the severity of symptoms in patients had a significant negative correlation with AD in the left cingulum bundle, superior longitudinal fasciculus, and bilateral posterior limb of internal capsule (Silk et al., 2013). A study showed that MD values in the right and left, anterior and posterior cerebellum were significantly correlated with specific alleles and single-nucleotide polymorphisms in paediatric OCD, but the anterior lobe of the cerebellum and pons were correlated with contamination and washing symptoms (Lazaro et al., 2014). Grazioplene et al. found that FA in the inferior longitudinal fasciculus was negatively associated with general obsessive–compulsive symptoms psychopathology scores, but this association was positive for the superior longitudinal fasciculus (Grazioplene et al., 2022). Interestingly these FA changes had a trend of a positive association in the youngest quantile and a negative association in the oldest. a They also found a negative association between FA and Repetition/Checking symptoms in the corpus callosum and cortico-spinal tract.
Medication and DTI parameters
In Zarei et al. and Gruner et al. study, ROI analysis of specific regions showed no difference between medicated and unmedicated patients (Gruner et al., 2012; Zarei et al., 2011). Moreover, no significant effects of medication on FA value were found in Fitzgerald et al. study (Fitzgerald et al., 2014a, b). While Piras et al. did not find any WM changes in paediatric OCD, they reported that lower FA in the sagittal stratum of adult OCD patients was associated with a higher percentage of medicated patients which is an important confounder in the study (Piras et al., 2021a, b, c).
Genetic and DTI parameters
Gasso and colleagues investigated the association between the MD value of WM structure and specific genetic polymorphisms (Gasso et al., 2015). Results showed the existence of an association between specific polymorphisms in genes of glutamatergic, dopaminergic, and neurodevelopmental pathways with MD value especially discovered in the region of right and left anterior and posterior cerebellar lobes and in the lingual gyrus of the occipital lobe. As mentioned, anterior and posterior lobes of the cerebellum, pons, and lingual gyrus had significantly higher MD, AD, and RD values compared with HCs, suggesting an involvement of these regions in the pathophysiology of the paediatric OCD (Lazaro et al., 2014).
Various polymorphisms in OCD were reported in glutamate transporter gene (rs3087879 (SLC1A1)), dopamine transporter gene (rs4975646, SLC6A3), dopaminergic receptor D3 (rs3777679, DRD3), nerve growth factor receptor gene (rs734194, rs2072446, NGFR) and the cadherin 9 gene (rs6885387, CDH9). Rs3087879 polymorphism of SLC1A1 gene had a significant correlation with higher MD value in paediatric OCD patients, especially in those with major allele homozygous (GG) for SLC1A1, rs3087879. The association of two polymorphisms of SLC6A3 rs4975646 and DRD3 rs3773679 with the MD value of WM also supported the involvement of the dopaminergic system. They also showed polymorphisms of NGFR, rs734194 and rs2072446, and CDH9 rs6885387 in association with WM microstructure. However, no association between these polymorphisms was found with FA.
These results show that there are several dopamine-related polymorphisms and glutamate-related polymorphisms linked to OCD and imply a polygenic model of OCD in which several genes contribute subtly and gradually to the likelihood of developing the condition. Interestingly, these polymorphisms are related to WM alterations. These findings highlight the crucial roles of dopamine and glutamate and WM integrity in the pathophysiology of OCD and support the participation of cortico-striatal-thalamic-cortical bundles in this process. They also support the participation of tracts outside the orbitofronto-striatal circuit including the cerebellum and occipital lobe.
Models of OCD and DTI parameters
Studies included in this review support the classic model of orbitofronto-striatal circuit alterations in OCD, however, it is insufficient in explaining all OCD WM changes. These fifteen studies reported widespread alterations in WM tracts that are beyond orbitofronto-striatal circuit. They also reported the involvement of tracts in the temporal, parietal, and occipital lobes. Association fibers that anatomically connect areas of the brain classically known to be involved in OCD include the cingulum, uncinate fasciculus, and arcuate fasciculus. However, the superior longitudinal fasciculus, inferior longitudinal fasciculus, and inferior-fronto-occipital fasciculus also showed altered integrity in paediatric OCD (Ameis et al., 2016; Gruner et al., 2012; Jayarajan et al., 2012; Zarei et al., 2011). Overall, paediatric OCD patients had alterations in brain WM structure in three distinct networks: the first, involving the orbitofrontal circuits, the anterior cingulate bundle, and temporal poles; the second, including the postcentral and lingual gyri WM connections, and the third comprising a circuit made by connections between the thalamus and occipital regions.
Several structural alterations in the WM connecting the orbitofrontal cortex, anterior cingulate cortex, thalamus, and caudate nucleus in the included studies supported the classic orbitofronto-subcortical circuits model (Ameis et al., 2016; Fitzgerald et al., 2014a, b; Gasso et al., 2015; Lazaro et al., 2014; Piras et al., 2021a, b, c; Silk et al., 2013). OCD phenomenology can be explained by functional and anatomical activity in the orbitofrontal circuits. The caudate serves as a gate for the limbic and frontal cortices, the anterior cingulate cortex as an activity monitor and regulator, the orbitofrontal cortex as a monitor of proper conduct in social life, and the thalamus as an information filter (Nakao et al., 2014).
Structural changes outside the orbitofrontal circuits comprised areas on the dorsolateral frontal and parietal lobes that may be thought to reflect the dorsolateral prefronto-striatal circuit that involves cognitive networks like spatial or attentional cognition. Posterior areas, including the WM tracts in the parietal and occipital lobes and cerebellum, also displayed altered integrity (Gruner et al., 2012; Jayarajan et al., 2012; Pagliaccio et al., 2020, 2021; Tikoo et al., 2021). These regions are involved in cognitive tasks (Nakao et al., 2014). OCD was likely to have a complex pathophysiological condition if both the orbitofronto-striatum and prefronto-limbic-posterior circuits were involved.
Interestingly, studies observed that different paediatric OCD dimensions were linked to quite unique components inside and outside the frontostriatothalamic circuit. For instance, considering contamination/washing-related stimuli, patients showed substantially lower FA than controls in the midbrain, lentiform nucleus, insula, thalamus, and higher MD, AD, and RD in the cerebellum and pons, whereas when facing presenting harm and checking symptoms, patients showed significantly lower FA in the corpus callosum, cingulate gyrus, and caudate nucleus (Gruner et al., 2012; Lazaro et al., 2014).
The relationship between clinical symptoms, cognitive functions, and the brain may be shown by combining neuropsychological and neuroimaging approaches.
Discussion
In this work, we conducted a systematic review of DTI investigations to study WM structure patterns in paediatric OCD patients. We found that several subnetworks of the WM in OCD patients are significantly disrupted, especially the networks that connect the medial orbitofrontal regions, the thalamus, the temporal poles, and the occipital regions.
Fifteen studies were included in this review. The majority of the studies were single-center case–control studies and included OCD groups and healthy controls. Two studies recruited healthy individuals with and without OCS (Grazioplene et al., 2022; Pagliaccio et al., 2021). These two studies were adequately powered and looked at the neural changes in the early stages of the disease. Three studies included an additional group of patients besides OCD and healthy controls. These three studies suggested that DTI is useful for the evaluation and comparison of white matter pathology (Ameis et al., 2016; Tikoo et al., 2021; White et al., 2015). Studying WM microstructural changes to characterize functional connectivity changes has a potential impact on clinical decision-making. Three studies investigated structural connectivity along with other imaging techniques (Pagliaccio et al., 2021; Tikoo et al., 2021; Zarei et al., 2011).
Many studies in the paediatric OCD population found diffuse WM alterations predominantly in the corpus callosum, cingulum, arcuate fasciculus, uncinate fasciculus, inferior longitudinal fasciculus, superior longitudinal fasciculus, inferior fronto-occipital fasciculus, corticospinal tract, forceps minor and major and the cerebellum. WM tracts connecting the prefrontal cortex, striatum, globus pallidus, and thalamus are primarily disrupted in paediatric OCD, however, there are WM changes beyond these tracts. Neural tracts connecting fronto-occipital lobes, occipito-temporal lobes, and cerebellum to the brain stem as well as corpus callosum are also involved in paediatric OCD. These inconsistent findings in these studies might reflect differences in methodological approaches particularly DTI analysis or be part of the pathophysiology of the disease spectrum.
Corpus callosum is heavily involved in the lateralization of sensorimotor and cognitive brain processes (Hoptman & Davidson, 1994; van der Knaap & van der Ham, 2011; Walterfang & Velakoulis, 2014). Studies using DTI to investigate how FA and MD values change during corpus callosum development showed that increased FA and decreased MD correlated with age (Barnea-Goraly et al., 2005; Bashat et al., 2005; Lebel et al., 2010; Schmithorst et al., 2008). Longitudinal DTI studies may be the best way to assess changes in the corpus callosum where neurodevelopmental changes are of particular interest.
The cingulum bundle connects frontal, parietal, and medial temporal lobes and basal ganglia with the cingulate gyrus (Bubb et al., 2018) and is thought to play an important role in the emotional and social cognition adjustment (Fitzsimmons et al., 2020), two types of behaviour that are often affected in OCD. Changes in WM integrity of the frontal and parietal lobes are also of relevance to OCD behaviour. These lobes are directly connected and play an important role in sensorimotor integration, which feeds directly into judgment and decision making. Involvement of other major pathways such as superior longitudinal fasciculus, inferior longitudinal fasciculus, superior fronto-occipital fasciculus, inferior fronto-occipital fasciculus, and the uncinate fasciculus is also reported. These pathways are primate-specific WM tracts connecting almost the entire cerebral cortex (Hua et al., 2008).
The corticospinal tract mainly contains pyramidal tracts that control voluntary muscle movements (Lemon & Griffiths, 2005). Corticospinal tract alterations in paediatric OCD patients were observed in some studies (Ameis et al., 2016; Gruner et al., 2012; Zarei et al., 2011). Involvement of this tract might be relevant to soft motor signs that have been frequently observed in OCD and related disorders (Bolton et al., 1998; Dhuri & Parkar, 2016; Ekinci & Erkan Ekinci, 2020; Malhotra et al., 2017). Cerebellar involvement in OCD was reported in three studies (Gasso et al., 2015; Lazaro et al., 2014; Tikoo et al., 2021). The cerebellum's role in the pathogenesis of a number of neuropsychiatric illnesses has attracted growing interest in recent years (Haghshomar et al., 2022). Previous studies have shown that the cerebellum may be crucial in the pathogenesis of OCD, as evidenced by OCD patients' aberrant spontaneous cerebellar activity and impaired functional connectivity between the cerebellum and the cortico-striato-thalamo-cortical circuit (Zhang et al., 2019).
OCD in children and adolescents is likely to have a neurodevelopmental basis. This is supported by DTI studies in paediatric OCD which showed various changes in WM integrity (Fitzgerald et al., 2014a, b; Gruner et al., 2012; Zarei et al., 2011). In addition, paediatric OCD was associated with increased thalamic and striatal volume (Van den Heuvel et al., 2022). It appears that in the process of maturation and growth the risk of psychiatric disorders increases (Paus et al., 2008). In several white-matter areas, DTI investigations show an age-related decline in the directionality and an increase in the magnitude of water diffusion. Such alterations in DTI-derived metrics may signify that axons and/or their myelin sheaths are still maturing and changes in the myelination, are prominent in this period (Schmithorst et al., 2002). The most widely accepted explanation for the anatomical findings in the adolescent brain includes alterations in synapse pruning and myelination (Snook et al., 2005). Changing levels of hormones occur throughout development, and steroid hormones have an impact on neuronal activity and morphology (Sisk & Foster, 2004). Notably, Grazioplene showed WM fiber density and age have a positive association in the younger age range and a negative association in the higher age range. They also found that FA changes in distinct areas of the brain have positive associations in the younger age quantile and negative associations in the older age quantile. This highlights the role of maturation in WM alterations and can explain contradictory results of FA alterations in paediatric OCD patients. Besides the maturation process, paediatric OCD patients experience a longer disease duration due to an early onset. Piras and colleagues found that FA reduction in the sagittal striatum correlated with OCD disease duration (Piras et al., 2021a, b, c). The latter is contrary to other studies including those in this review. The reason for this inconsistency might be methodological in origin. Studies included in this review were mostly based on TBSS analysis. This method directly maps diffusion values from each subject onto a reference skeleton for group comparison, building a white-matter skeleton, which is restricted to the center of WM pathways, in order to minimize the possible misalignment that may occur in voxel-based whole-brain analysis (Smith et al., 2006). The analysis of multi-subject diffusion imaging investigations is enhanced by TBSS in terms of sensitivity, objectivity, and interpretability. Besides different imaging protocols, clinical characterization of the cohorts and comorbidities may also be important confounding variables (Table 1) contributing to inconsistent findings.
Sex differences is also an important factor: there are differences in FA map of female vs male brain (Schmithorst et al., 2008). The interaction between sex and OCD subtypes is also a factor (den Braber et al., 2013), particularly that OCD is more common in males than females in childhood, but this propensity reverses in adulthood.
Another explanation for inconsistent results of DTI studies on paediatric OCD is the heterogeneity of OCD symptoms. OCD is a highly heterogeneous mental illness and individuals with the same diagnosis of OCD might come with completely different, non-overlapping obsessions or/and compulsions characteristics. There is a great variability in symptoms and severity of OCD, measured by the Yale-Brown Obsessive–Compulsive Scale (Y-BOCS) score. Mild symptoms are between 0–13, moderate 14–25, moderate-severe 26–34, and severe symptoms by 35–40 (Storch et al., 2015). Y-BOCS values in the DTI studies were between 10–28. Most patients had various and multiple obsessive and/or compulsive symptoms (OCS) and only a minority were monosymptomatic. A previous meta-analysis identified four OCS dimensions: contamination and washing; symmetry and arranging; banned ideas, and checking; and hoarding (Bloch et al., 2008).
We found that each OCS dimension might be connected to a particular neurobiological substrate. Based on the studies in this review contamination and washing-related symptoms were related to WM alterations within and also outside the frontostriatothalamic circuits while checking symptoms were associated with the main altered tracts in OCD including the corpus callosum and cingulate gyrus. Recent fMRI studies utilizing neuropsychological tasks during fMRI have shown a connection between cognitive impairment and clinical symptoms in OCD (Nakao et al., 2014).
A functional MRI study showed different patterns of cortical activation in relation to different categories of OCD symptoms; Mataix-Cols found that OCD patients showed significantly greater activation than controls in the thalamus, putamen/globus pallidus, and dorsal cortical regions when exposed to contamination and washing-related stimuli. In contrast, patients showed significantly greater activation than controls in the right caudate nucleus and bilateral ventromedial prefrontal regions when exposed to banned ideas and checking-related stimuli, (Mataix-Cols et al., 2008). Van den Heuvel et al. sought to examine variations in the volumes of different brain regions' white and grey matter and came to the conclusion that people with symmetry dimension symptoms had a smaller right motor cortex volume (Van Den Heuvel et al., 2009). Similar to this, Alvarenga et al. found that those with higher aggressiveness ratings had larger lateral parietal cortex sizes in both hemispheres, whereas people with higher sexual/religious dimension volumes had larger insula volumes in both hemispheres (Alvarenga et al., 2012).
This dimensional model of OCD has its limitations. Phenotypic data is used to build this model. Even if the stated symptomatology is of utmost significance in psychiatry, it might be deceptive to categorize people just by their symptoms. Different processes underlie the symptoms of OCS. Some large neuroimaging studies using mega-analysis approaches have failed to identify different neuroanatomical correlations for each OCS dimension (Boedhoe et al., 2017, 2018). This issue needs to be addressed in future studies.
Another factor is the effect of long-term pharmacotherapy, which may affect DTI measures (Insel et al., 2008; Wang et al., 2013). The majority of the patients in this review were on SSRI treatment. SSRIs were shown to affect diffusion measures (Seiger et al., 2021). SSRIs may also affect brain development by increasing Brain-Derived Neurotrophic Factor (Hunsberger et al., 2009), through its effect on oligodendrocytes (Xiao et al., 2010), and astrocytic glycogenolysis (Sijens et al., 2008). These changes may potentially alter diffusivity coefficients. Some paediatric patients with severe OCD were on antipsychotics. These drugs may also affect DTI measures, for example by reducing the number of glial cells and myelination process (Alexander et al., 2011; Konopaske et al., 2008). Clearly, the effect of medication could be a potential source of variability in DTI studies in paediatric OCD. What makes the effect of drugs on DTI measures even more complex is that this effect is unpredictable and variable between diseases and individuals (Sagarwala & Nasrallah, 2020). Taken together the exact effects of medication on WM changes are not fully understood and future studies should take this effect into account.
Finally, the result of this systematic review is limited by the low number of studies included, as well as differences in study design, imaging protocol, symptomatology, small sample sizes, and image analysis approach. A large prospective longitudinal study using multimodal structural and functional imaging methods from early childhood well into adulthood in a well-powered and clinically characterized cohort is required to understand the neural substrates of OCD and its relationship with growth and development. As studies show FA changes vastly during maturation, a recommendation for a future study is to investigate WM changes in distinct age groups of paediatric OCD patients.
Conclusion
DTI is a useful tool for a deeper understanding of microstructural changes in the brain particularly in the WM. DTI studies of children with OCD demonstrated altered integrity in various anatomical connections. This technique together with other neuroimaging methods may play a vital role in our understanding of OCD. However, various factors affect the result of DTI analysis and therefore its interpretation should be considered with caution and with a full understanding of methodological issues and in the context of clinical information.
Data availability
Not applicable.
Code availability
Not applicable.
References
Abdolalizadeh, A., Ostadrahimi, H., Ohadi, M. A. D., Saneei, S. A., & Ershadi, A. S. B. (2021). White matter microstructural associates of apathy-avolition in schizophrenia. Journal of Psychiatric Research, 142, 110–116.
Alexander, A. L., Hurley, S. A., Samsonov, A. A., Adluru, N., Hosseinbor, A. P., Mossahebi, P., . . . Field, A. S. (2011). Characterization of cerebral white matter properties using quantitative magnetic resonance imaging stains. Brain Connectivity, 1(6), 423–446.
Alvarenga, P. G., do Rosário, M. C., Batistuzzo, M. C., Diniz, J. B., Shavitt, R. G., Duran, F. L., . . . Hoexter, M. Q. (2012). Obsessive-compulsive symptom dimensions correlate to specific gray matter volumes in treatment-naïve patients. Journal of Psychiatric Research, 46(1), 1635–1642.
Ameis, S. H., Lerch, J. P., Taylor, M. J., Lee, W., Viviano, J. D., Pipitone, J., . . . Anagnostou, E. (2016). A diffusion tensor imaging study in children with ADHD, autism spectrum disorder, OCD, and matched controls: Distinct and non-distinct white matter disruption and dimensional brain-behavior relationships. American Journal of Psychiatry, 173(12), 1213–1222. https://doi.org/10.1176/appi.ajp.2016.15111435
Ashraf-Ganjouei, A., Rahmani, F., Aarabi, M. H., Sanjari Moghaddam, H., Nazem-Zadeh, M.-R., Davoodi-Bojd, E., & Soltanian-Zadeh, H. (2019). White matter correlates of disease duration in patients with temporal lobe epilepsy: Updated review of literature. Neurological Sciences, 40(6), 1209–1216.
Barnea-Goraly, N., Menon, V., Eckert, M., Tamm, L., Bammer, R., Karchemskiy, A., . . . Reiss, A. L. (2005). White Matter development during childhood and adolescence: A cross-sectional diffusion tensor imaging study. Cerebral Cortex, 15(12), 1848–1854. https://doi.org/10.1093/cercor/bhi062
Bashat, D. B., Sira, L. B., Graif, M., Pianka, P., Hendler, T., Cohen, Y., & Assaf, Y. (2005). Normal white matter development from infancy to adulthood: Comparing diffusion tensor and high b value diffusion weighted MR images. Journal of Magnetic Resonance Imaging, 21(5), 503–511. https://doi.org/10.1002/jmri.20281
Benedetti, F., Giacosa, C., Radaelli, D., Poletti, S., Pozzi, E., Dallaspezia, S., . . . Smeraldi, E. (2013). Widespread changes of white matter microstructure in obsessive–compulsive disorder: Effect of drug status. European Neuropsychopharmacology, 23(7), 581–593.
Bennett, I. J., Madden, D. J., Vaidya, C. J., Howard, D. V., & Howard, J. H., Jr. (2010). Age-related differences in multiple measures of white matter integrity: A diffusion tensor imaging study of healthy aging. Human Brain Mapping, 31(3), 378–390.
Bloch, M. H., Landeros-Weisenberger, A., Rosario, M. C., Pittenger, C., & Leckman, J. F. (2008). Meta-analysis of the symptom structure of obsessive-compulsive disorder. American Journal of Psychiatry, 165(12), 1532–1542. https://doi.org/10.1176/appi.ajp.2008.08020320
Boedhoe, P. S., Schmaal, L., Abe, Y., Ameis, S. H., Arnold, P. D., Batistuzzo, M. C., . . . Bose, A. (2017). Distinct subcortical volume alterations in pediatric and adult OCD: a worldwide meta-and mega-analysis. American Journal of Psychiatry, 174(1), 60–69.
Boedhoe, P. S., Schmaal, L., Abe, Y., Alonso, P., Ameis, S. H., Anticevic, A., . . . Beucke, J. C. (2018). Cortical abnormalities associated with pediatric and adult obsessive-compulsive disorder: findings from the ENIGMA Obsessive-Compulsive Disorder Working Group. American Journal of Psychiatry, 175(5), 453–462.
Bolton, D., Gibb, W., Lees, A., Raven, P., Gray, J. A., Chen, E., & Shafran, R. (1998). Neurological soft signs in obsessive compulsive disorder: Standardised assessment and comparison with schizophrenia. Behavioural Neurology, 11(4), 197–204. https://doi.org/10.1155/1999/639045
Bosch, B., Arenaza-Urquijo, E. M., Rami, L., Sala-Llonch, R., Junqué, C., Solé-Padullés, C., . . . Bartrés-Faz, D. (2012). Multiple DTI index analysis in normal aging, amnestic MCI and AD. Relationship with neuropsychological performance. Neurobiology of Aging, 33(1), 61–74.
Brouwer, R. M., Mandl, R. C., Schnack, H. G., van Soelen, I. L., van Baal, G. C., Peper, J. S., . . . Pol, H. H. (2012). White matter development in early puberty: a longitudinal volumetric and diffusion tensor imaging twin study. PloS one, 7(4), e32316.
Bubb, E. J., Metzler-Baddeley, C., & Aggleton, J. P. (2018). The cingulum bundle: Anatomy, function, and dysfunction. Neuroscience and Biobehavioral Reviews, 92, 104–127. https://doi.org/10.1016/j.neubiorev.2018.05.008
Chamberlain, S. R., Menzies, L., Hampshire, A., Suckling, J., Fineberg, N. A., del Campo, N., . . . Bullmore, E. T. (2008). Orbitofrontal dysfunction in patients with obsessive-compulsive disorder and their unaffected relatives. Science, 321(5887), 421–422.
Christian, C. J., Lencz, T., Robinson, D. G., Burdick, K. E., Ashtari, M., Malhotra, A. K., . . . Szeszko, P. R. (2008). Gray matter structural alterations in obsessive–compulsive disorder: Relationship to neuropsychological functions. Psychiatry Research: Neuroimaging, 164(2), 123–131.
Correia, S., Lee, S. Y., Voorn, T., Tate, D. F., Paul, R. H., Zhang, S., . . . Laidlaw, D. H. (2008). Quantitative tractography metrics of white matter integrity in diffusion-tensor MRI. Neuroimage, 42(2), 568–581.
DeBoy, C. A., Zhang, J., Dike, S., Shats, I., Jones, M., Reich, D. S., . . . Miller, R. H. (2007). High resolution diffusion tensor imaging of axonal damage in focal inflammatory and demyelinating lesions in rat spinal cord. Brain, 130(8), 2199–2210.
den Braber, A., de Geus, E. J., & Boomsmavan‘t Ent, D. I. D. (2013). Obsessive–compulsive symptoms and related sex differences in brain structure: An MRI study in Dutch twins. Twin Research and Human Genetics, 16(2), 516–524.
Dhuri, C. V., & Parkar, S. R. (2016). Soft neurological signs and cognitive function in obsessive-compulsive disorder patients. Indian Journal of Psychological Medicine, 38(4), 291–295. https://doi.org/10.4103/0253-7176.185957
Drubach, D. A. (2015). Obsessive-compulsive disorder. CONTINUUM: Lifelong Learning in Neurology, 21(3), 783–788. https://doi.org/10.1212/01.Con.0000466666.12779.07
Ekinci, O., & ErkanEkinci, A. (2020). Neurological soft signs and clinical features of tic-related obsessive-compulsive disorder indicate a unique subtype. The Journal of Nervous and Mental Disease, 208(1), 21–27. https://doi.org/10.1097/NMD.0000000000001098
Eslinger, P. J., & Damasio, A. R. (1985). Severe disturbance of higher cognition after bilateral frontal lobe ablation: Patient EVR. Neurology, 35(12), 1731–1731.
Ferreira, S., Pêgo, J. M., & Morgado, P. (2020). A systematic review of behavioral, physiological, and neurobiological cognitive regulation alterations in obsessive-compulsive disorder. Brain Sciences. https://doi.org/10.3390/brainsci10110797
Fitzgerald, K. D., Liu, Y., Reamer, E. N., Taylor, S. F., & Welsh, R. C. (2014a). Atypical frontal-striatal-thalamic circuit white matter development in pediatric obsessive-compulsive disorder. Journal of the American Academy of Child and Adolescent Psychiatry, 53(11), 1225–1233. https://doi.org/10.1016/j.jaac.2014.08.010. 1233.e1221-1229.
Fitzgerald, K. D., Liu, Y., Reamer, E. N., Taylor, S. F., & Welsh, R. C. (2014b). Atypical frontal–striatal–thalamic circuit white matter development in pediatric obsessive-compulsive disorder. Journal of the American Academy of Child & Adolescent Psychiatry, 53(11), 1225-1233. e1229.
Fitzsimmons, J., Rosa, P., Sydnor, V. J., Reid, B. E., Makris, N., Goldstein, J. M., . . . , Kubicki, M. (2020). Cingulum bundle abnormalities and risk for schizophrenia. Schizophrenia Research, 215, 385–391. https://doi.org/10.1016/j.schres.2019.08.017
Frydman, I., de Salles Andrade, J. B., Vigne, P., & Fontenelle, L. F. (2016). Can neuroimaging provide reliable biomarkers for obsessive-compulsive disorder? A Narrative Review. Curr Psychiatry Rep, 18(10), 90. https://doi.org/10.1007/s11920-016-0729-7
Gan, J., Zhong, M., Fan, J., Liu, W., Niu, C., Cai, S., . . . Zhu, X. (2017). Abnormal white matter structural connectivity in adults with obsessive-compulsive disorder. Translational Psychiatry, 7(3), e1062. https://doi.org/10.1038/tp.2017.22
Gasso, P., Ortiz, A. E., Mas, S., Morer, A., Calvo, A., Bargallo, N., . . . Lazaro, L. (2015). Association between genetic variants related to glutamatergic, dopaminergic and neurodevelopment pathways and white matter microstructure in child and adolescent patients with obsessive-compulsive disorder. Journal of Affective Disorders, 186, 284–292. https://doi.org/10.1016/j.jad.2015.07.035
Ghazi Sherbaf, F., Mojtahed Zadeh, M., Haghshomar, M., & Aarabi, M. H. (2019). Posterior limb of the internal capsule predicts poor quality of life in patients with Parkinson’s disease: Connectometry approach. Acta Neurologica Belgica, 119(1), 95–100.
Giorgio, A., Watkins, K. E., Chadwick, M., James, S., Winmill, L., Douaud, G., . . . Johansen-Berg, H. (2010). Longitudinal changes in grey and white matter during adolescence. Neuroimage, 49(1), 94–103.
Grazioplene, R. G., DeYoung, C. G., Hampson, M., Anticevic, A., & Pittenger, C. (2022). Obsessive compulsive symptom dimensions are linked to altered white-matter microstructure in a community sample of youth. Translational Psychiatry, 12(1), 1–10.
Gruner, P., Vo, A., Ikuta, T., Mahon, K., Peters, B. D., Malhotra, A. K., . . . Szeszko, P. R. (2012). White matter abnormalities in pediatric obsessive-compulsive disorder. Neuropsychopharmacology, 37(12), 2730–2739. https://doi.org/10.1038/npp.2012.138
Haghshomar, M., Shobeiri, P., Seyedi, S. A., Abbasi-Feijani, F., Poopak, A., Sotoudeh, H., & Kamali, A. (2022). Cerebellar microstructural abnormalities in Parkinson’s disease: A systematic review of diffusion tensor imaging studies. The Cerebellum, 1–27.
Hoptman, M. J., & Davidson, R. J. (1994). How and why do the two cerebral hemispheres interact? Psychological Bulletin, 116(2), 195–219. https://doi.org/10.1037/0033-2909.116.2.195
Hu, X., Zhang, L., Bu, X., Li, H., Gao, Y., Lu, L., . . . Gong, Q. (2020). White matter disruption in obsessive-compulsive disorder revealed by meta-analysis of tract-based spatial statistics. Depress Anxiety, 37(7), 620–631. https://doi.org/10.1002/da.23008
Hua, K., Oishi, K., Zhang, J., Wakana, S., Yoshioka, T., Zhang, W., . . . Mori, S. (2008). Mapping of functional areas in the human cortex based on connectivity through association fibers. Cerebral Cortex, 19(8), 1889–1895. https://doi.org/10.1093/cercor/bhn215
Hunsberger, J., Austin, D. R., Henter, I. D., & Chen, G. (2009). The neurotrophic and neuroprotective effects of psychotropic agents. Dialogues in Clinical Neuroscience, 11(3), 333.
Insel, K. C., Reminger, S. L., & Hsiao, C.-P. (2008). White matter hyperintensities and medication adherence. Biological Research for Nursing, 10(2), 121–127.
Jayarajan, R. N., Venkatasubramanian, G., Viswanath, B., Janardhan Reddy, Y. C., Srinath, S., Vasudev, M. K., & Chandrashekar, C. R. (2012). White matter abnormalities in children and adolescents with obsessive-compulsive disorder: A diffusion tensor imaging study. Depression and Anxiety, 29(9), 780–788. https://doi.org/10.1002/da.21890
Jeurissen, B., Descoteaux, M., Mori, S., & Leemans, A. (2019). Diffusion MRI fiber tractography of the brain. NMR in Biomedicine, 32(4), e3785.
Jones, D. K., Symms, M. R., Cercignani, M., & Howard, R. J. (2005). The effect of filter size on VBM analyses of DT-MRI data. NeuroImage, 26(2), 546–554.
Koch, K., Reeß, T. J., Rus, O. G., Zimmer, C., & Zaudig, M. (2014). Diffusion tensor imaging (DTI) studies in patients with obsessive-compulsive disorder (OCD): A review. Journal of Psychiatric Research, 54, 26–35.
Konopaske, G. T., Dorph-Petersen, K.-A., Sweet, R. A., Pierri, J. N., Zhang, W., Sampson, A. R., & Lewis, D. A. (2008). Effect of chronic antipsychotic exposure on astrocyte and oligodendrocyte numbers in macaque monkeys. Biological Psychiatry, 63(8), 759–765.
Lazaro, L., Calvo, A., Ortiz, A. G., Ortiz, A. E., Morer, A., Moreno, E.,. . . . , Bargallo, N. (2014). Microstructural brain abnormalities and symptom dimensions in child and adolescent patients with obsessive-compulsive disorder: a diffusion tensor imaging study. Depress Anxiety, 31(12), 1007–1017. https://doi.org/10.1002/da.22330
Lebel, C., Caverhill-Godkewitsch, S., & Beaulieu, C. (2010). Age-related regional variations of the corpus callosum identified by diffusion tensor tractography. NeuroImage, 52(1), 20–31. https://doi.org/10.1016/j.neuroimage.2010.03.072
Lemon, R. N., & Griffiths, J. (2005). Comparing the function of the corticospinal system in different species: Organizational differences for motor specialization? Muscle and Nerve, 32(3), 261–279. https://doi.org/10.1002/mus.20333
Magioncalda, P., Martino, M., Ely, B. A., Inglese, M., & Stern, E. R. (2016). Microstructural white-matter abnormalities and their relationship with cognitive dysfunction in obsessive-compulsive disorder. Brain and Behavior, 6(3), e00442. https://doi.org/10.1002/brb3.442
Malhotra, D. S., Borade, D. P., Sharma, D. P., Satija, D. Y., & Dr, G. (2017). A qualititative study of neurological soft signs in obsessive compulsive disorder and effect of comorbid psychotic spectrum disorders and familiality on its expression in Indian population. Asian Journal of Psychiatry, 25, 6–12. https://doi.org/10.1016/j.ajp.2016.06.020
Mataix-Cols, D., Nakatani, E., Micali, N., & Heyman, I. (2008). Structure of obsessive-compulsive symptoms in pediatric OCD. Journal of the American Academy of Child & Adolescent Psychiatry, 47(7), 773–778. https://doi.org/10.1097/CHI.0b013e31816b73c0
Mavridis, D., Chaimani, A., Efthimiou, O., & Salanti, G. (2018). Missing outcome data in meta-analysis. Evidence-Based Mental Health, 21(3), 123. https://doi.org/10.1136/eb-2014-101899
Menzies, L., Chamberlain, S. R., Laird, A. R., Thelen, S. M., Sahakian, B. J., & Bullmore, E. T. (2008a). Integrating evidence from neuroimaging and neuropsychological studies of obsessive-compulsive disorder: The orbitofronto-striatal model revisited. Neuroscience & Biobehavioral Reviews, 32(3), 525–549.
Menzies, L., Williams, G. B., Chamberlain, S. R., Ooi, C., Fineberg, N., Suckling, J., . . . Bullmore, E. T. (2008b). White matter abnormalities in patients with obsessive-compulsive disorder and their first-degree relatives. American Journal of Psychiatry, 165(10), 1308–1315. https://doi.org/10.1176/appi.ajp.2008.07101677
Nakao, T., Okada, K., & Kanba, S. (2014). Neurobiological model of obsessive–compulsive disorder: Evidence from recent neuropsychological and neuroimaging findings. Psychiatry and Clinical Neurosciences, 68(8), 587–605.
Pagliaccio, D., Durham, K., Fitzgerald, K. D., & Marsh, R. (2021). Obsessive-compulsive symptoms among children in the adolescent brain and cognitive development study: Clinical, cognitive, and brain connectivity correlates. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 6(4), 399–409.
Pagliaccio, D., Cha, J., He, X., Cyr, M., Yanes-Lukin, P., Goldberg, P., Fontaine, M., Rynn, M. A., Marsh, R. (2020). Structural neural markers of response to cognitive behavioral therapy in pediatric obsessive-compulsive disorder. Journal of Child Psychology and Psychiatry, 61(12), 1299–1308. https://doi.org/10.1111/jcpp.13191
Paus, T., Keshavan, M., & Giedd, J. N. (2008). Why do many psychiatric disorders emerge during adolescence? Nature Reviews Neuroscience, 9(12), 947–957.
Peterson, J., Welch, V., Losos, M., & Tugwell, P. (2011). The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses (pp. 1–12). Ottawa Hospital Research Institute.
Piras, F., Piras, F., Caltagirone, C., & Spalletta, G. (2013). Brain circuitries of obsessive compulsive disorder: A systematic review and meta-analysis of diffusion tensor imaging studies. Neuroscience & Biobehavioral Reviews, 37(10), 2856–2877.
Piras, F., Piras, F., Chiapponi, C., Girardi, P., Caltagirone, C., & Spalletta, G. (2015). Widespread structural brain changes in OCD: A systematic review of voxel-based morphometry studies. Cortex, 62, 89–108. https://doi.org/10.1016/j.cortex.2013.01.016
Piras, F., Vecchio, D., Kurth, F., Piras, F., Banaj, N., Ciullo, V., & Spalletta, G. (2021c). Corpus callosum morphology in major mental disorders: a magnetic resonance imaging study. Brain Communications, 3(2), fcab100.
Piras, F., Piras, F., Abe, Y., Agarwal, S. M., Anticevic, A., Ameis, S., . . . Batistuzzo, M. C. (2021a). White matter microstructure and its relation to clinical features of obsessive–compulsive disorder: findings from the ENIGMA OCD Working Group. Translational Psychiatry, 11(1), 1–11.
Piras, F., Piras, F., Abe, Y., Agarwal, S. M., Anticevic, A., Ameis, S., . . . Spalletta, G. (2021b). White matter microstructure and its relation to clinical features of obsessive-compulsive disorder: findings from the ENIGMA OCD Working Group. Translational Psychiatry, 11(1), 173. https://doi.org/10.1038/s41398-021-01276-z
Rosso, I. M., Olson, E. A., Britton, J. C., Stewart, S. E., Papadimitriou, G., Killgore, W. D., . . . Rauch, S. L. (2014). Brain white matter integrity and association with age at onset in pediatric obsessive-compulsive disorder. Biology of Mood & Anxiety Disorders, 4(1), 13. https://doi.org/10.1186/s13587-014-0013-6
Rus, O. G., Reess, T. J., Wagner, G., Zimmer, C., Zaudig, M., & Koch, K. (2017). Functional and structural connectivity of the amygdala in obsessive-compulsive disorder. Neuroimage Clin, 13, 246–255. https://doi.org/10.1016/j.nicl.2016.12.007
Sagarwala, R., & Nasrallah, H. A. (2020). A systematic review of diffusion tensor imaging studies in drug-naïve OCD patients before and after pharmacotherapy. Annals of Clinical Psychiatry: Official Journal of the American Academy of Clinical Psychiatrists, 32(1), 42–47.
Schmithorst, V. J., Wilke, M., Dardzinski, B. J., & Holland, S. K. (2002). Correlation of white matter diffusivity and anisotropy with age during childhood and adolescence: A cross-sectional diffusion-tensor MR imaging study. Radiology, 222(1), 212.
Schmithorst, V. J., Holland, S. K., & Dardzinski, B. J. (2008). Developmental differences in white matter architecture between boys and girls. Human Brain Mapping, 29(6), 696–710. https://doi.org/10.1002/hbm.20431
Seiger, R., Gryglewski, G., Klobl, M., Kautzky, A., Godbersen, G. M., Rischka, L., . . . Lanzenberger, R. (2021). The influence of acute SSRI administration on white matter microstructure in patients suffering from major depressive disorder and healthy controls. International Journal of Neuropsychopharmacology, 24(7), 542–550. https://doi.org/10.1093/ijnp/pyab008
Seyedmirzaei, H., Shafie, M., Kargar, A., Golbahari, A., Bijarchian, M., Ahmadi, S., . . . Mayeli, M. (2022). White matter tracts associated with alexithymia and emotion regulation: A diffusion MRI study. Journal of Affective Disorders, 314, 271–280.
Sijens, P. E., Mostert, J. P., Irwan, R., Potze, J. H., Oudkerk, M., & De Keyser, J. (2008). Impact of fluoxetine on the human brain in multiple sclerosis as quantified by proton magnetic resonance spectroscopy and diffusion tensor imaging. Psychiatry Research: Neuroimaging, 164(3), 274–282.
Silk, T., Chen, J., Seal, M., & Vance, A. (2013). White matter abnormalities in pediatric obsessive-compulsive disorder. Psychiatry Research, 213(2), 154–160. https://doi.org/10.1016/j.pscychresns.2013.04.003
Sisk, C. L., & Foster, D. L. (2004). The neural basis of puberty and adolescence. Nature Neuroscience, 7(10), 1040–1047.
Smith, S. M., Jenkinson, M., Johansen-Berg, H., Rueckert, D., Nichols, T. E., Mackay, C. E., . . . Matthews, P. M. (2006). Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage, 31(4), 1487–1505.
Snook, L., Paulson, L.-A., Roy, D., Phillips, L., & Beaulieu, C. (2005). Diffusion tensor imaging of neurodevelopment in children and young adults. NeuroImage, 26(4), 1164–1173.
Song, S. K., Sun, S. W., Ju, W. K., Lin, S. J., Cross, A. H., & Neufeld, A. H. (2003). Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. NeuroImage, 20(3), 1714–1722. https://doi.org/10.1016/j.neuroimage.2003.07.005
Stein, D. J. (2002). Obsessive-compulsive disorder. The Lancet, 360(9330), 397–405.
Storch, E. A., De Nadai, A. S., Do Rosário, M. C., Shavitt, R. G., Torres, A. R., Ferrão, Y. A., . . . Fontenelle, L. F. (2015). Defining clinical severity in adults with obsessive–compulsive disorder. Comprehensive psychiatry, 63, 30–35.
Tikoo, S., Suppa, A., Tommasin, S., Giannì, C., Conte, G., Mirabella, G., . . . Pantano, P. (2021). The cerebellum in drug-naive children with tourette syndrome and obsessive–compulsive disorder. The Cerebellum, 1–12.
Togao, O., Yoshiura, T., Nakao, T., Nabeyama, M., Sanematsu, H., Nakagawa, A., . . . Honda, H. (2010). Regional gray and white matter volume abnormalities in obsessive-compulsive disorder: a voxel-based morphometry study. Psychiatry Research, 184(1), 29–37. https://doi.org/10.1016/j.pscychresns.2010.06.011
Van Den Heuvel, O. A., Remijnse, P. L., Mataix-Cols, D., Vrenken, H., Groenewegen, H. J., Uylings, H. B., . . . Veltman, D. J. (2009). The major symptom dimensions of obsessive-compulsive disorder are mediated by partially distinct neural systems. Brain, 132(4), 853–868.
Van den Heuvel, O. A., Boedhoe, P. S., Bertolin, S., Bruin, W. B., Francks, C., Ivanov, I., . . . O'Neill, J. (2022). An overview of the first 5 years of the ENIGMA obsessive–compulsive disorder working group: The power of worldwide collaboration. Human Brain Mapping, 43(1), 23–36.
van der Knaap, L. J., & van der Ham, I. J. (2011). How does the corpus callosum mediate interhemispheric transfer? A Review. Behav Brain Res, 223(1), 211–221. https://doi.org/10.1016/j.bbr.2011.04.018
Walterfang, M., & Velakoulis, D. (2014). Callosal morphology in schizophrenia: What can shape tell us about function and illness? British Journal of Psychiatry, 204(1), 9–11. https://doi.org/10.1192/bjp.bp.113.132357
Wang, Q., Cheung, C., Deng, W., Li, M., Huang, C., Ma, X., . . . Collier, D. (2013). White-matter microstructure in previously drug-naive patients with schizophrenia after 6 weeks of treatment. Psychological Medicine, 43(11), 2301–2309.
Weeland, C. J., Kasprzak, S., De Joode, N. T., Abe, Y., Alonso, P., Ameis, S. H., . . . Banaj, N. (2022). The thalamus and its subnuclei—a gateway to obsessive-compulsive disorder. Translational Psychiatry, 12(1), 1–10.
White, T., Langen, C., Schmidt, M., Hough, M., & James, A. (2015). Comparative neuropsychiatry: White matter abnormalities in children and adolescents with schizophrenia, bipolar affective disorder, and obsessive-compulsive disorder. European Psychiatry, 30(2), 205–213.
Xiao, J., Wong, A. W., Willingham, M. M., van den Buuse, M., Kilpatrick, T. J., & Murray, S. S. (2010). Brain-derived neurotrophic factor promotes central nervous system myelination via a direct effect upon oligodendrocytes. Neurosignals, 18(3), 186–202.
Zarei, M., Mataix-Cols, D., Heyman, I., Hough, M., Doherty, J., Burge, L., . . . James, A. (2011). Changes in gray matter volume and white matter microstructure in adolescents with obsessive-compulsive disorder. Biological Psychiatry, 70(11), 1083–1090.
Zhang, H., Wang, B., Li, K., Wang, X., Li, X., Zhu, J., . . . Zhang, M. (2019). Altered functional connectivity between the cerebellum and the cortico-striato-thalamo-cortical circuit in obsessive-compulsive disorder. Frontiers in Psychiatry, 10, 522.
Zhou, C., Xu, J., Ping, L., Zhang, F., Chen, W., Shen, Z., . . . Cheng, Y. (2018). Cortical thickness and white matter integrity abnormalities in obsessive-compulsive disorder: A combined multimodal surface-based morphometry and tract-based spatial statistics study. Depress Anxiety, 35(8), 742–751. https://doi.org/10.1002/da.22758
Acknowledgements
We sincerely thank the authors of the included articles in this systematic review due to sharing the relevant data.
Funding
MZ was supported by Lundbeck Foundation.
Author information
Authors and Affiliations
Contributions
Conceptualization: MH, SPM, MZ;
Literature search and data extraction: MH, SPM;
Writing—original draft preparation: MH, SPM;
Writing – review, and editing: MZ, AJ, PS
All the authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflicts of interest/Competing interests
There were no conflicts of interest or competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Haghshomar, M., Mirghaderi, S.P., Shobeiri, P. et al. White matter abnormalities in paediatric obsessive–compulsive disorder: a systematic review of diffusion tensor imaging studies. Brain Imaging and Behavior 17, 343–366 (2023). https://doi.org/10.1007/s11682-023-00761-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-023-00761-x