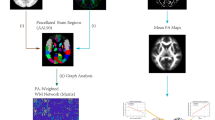
Abstract
Unilateral temporal lobe epilepsy (TLE) is the most common type of focal epilepsy characterized by foci in the unilateral temporal lobe grey matters of regions such as the hippocampus. However, it remains unclear how the functional features of white matter are altered in TLE. In the current study, resting-state functional magnetic resonance imaging (fMRI) was performed on 71 left TLE (LTLE) patients, 79 right TLE (RTLE) patients and 47 healthy controls (HC). Clustering analysis was used to identify fourteen white matter networks (WMN). The functional connectivity (FC) was calculated among WMNs and between WMNs and grey matter. Furthermore, the FC laterality of hemispheric WMNs was assessed. First, both patient groups showed decreased FCs among WMNs. Specifically, cerebellar white matter illustrated decreased FCs with the cerebral superficial WMNs, implying a dysfunctional interaction between the cerebellum and the cerebral cortex in TLE. Second, the FCs between WMNs and the ipsilateral hippocampus (grey matter foci) were also reduced in patient groups, which may suggest insufficient functional integration in unilateral TLE. Interestingly, RTLE showed more severe abnormalities of white matter FCs, including links to the bilateral hippocampi and temporal white matter, than LTLE. Taken together, these findings provide functional evidence of white matter abnormalities, extending the understanding of the pathological mechanism of white matter impairments in unilateral TLE.






Similar content being viewed by others
References
Agcaoglu, O., et al. (2015). Lateralization of resting state networks and relationship to age and gender. NeuroImage, 104, 310–325.
Bellec, P., et al. (2010). Multi-level bootstrap analysis of stable clusters in resting-state fMRI. NeuroImage, 51(3), 1126–1139.
Bernhardt, B. C., et al. (2016). The spectrum of structural and functional imaging abnormalities in temporal lobe epilepsy. Annals of Neurology, 80(1), 142–153.
Bonilha, L., et al. (2004). Voxel-based morphometry reveals gray matter network atrophy in refractory medial temporal lobe epilepsy. Archives of Neurology, 61(9), 1379–1384.
Cataldi, M., Avoli, M., & de Villers-Sidani, E. (2013). Resting state networks in temporal lobe epilepsy. Epilepsia, 54(12), 2048–2059.
Craddock, R. C., et al. (2012). A whole brain fMRI atlas generated via spatially constrained spectral clustering. Human Brain Mapping, 33(8), 1914–1928.
DeSalvo, M. N., et al. (2014). Altered structural connectome in temporal lobe epilepsy. Radiology, 270(3), 842–848.
Desikan, R. S., et al. (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage, 31(3), 968–980.
Diehl, B., et al. (2008). Abnormalities in diffusion tensor imaging of the uncinate fasciculus relate to reduced memory in temporal lobe epilepsy. Epilepsia, 49(8), 1409–1418.
Ding, Z. H., et al. (2018). Detection of synchronous brain activity in white matter tracts at rest and under functional loading. Proceedings of the National Academy of Sciences of the United States of America, 115(3), 595–600.
Dupont, S., et al. (2000). Episodic memory in left temporal lobe epilepsy: A functional MRI study. Brain, 123, 1722–1732.
Engel, J., Jr., & International League Against Epilepsy. (2001) A proposed diagnostic scheme for people with epileptic seizures and with epilepsy: Report of the ILAE Task Force on Classification and Terminology. Epilepsia, 42(6), 796–803.
Fabri, M., et al. (2011). Topographical organization of human corpus callosum: An fMRI mapping study. Brain Research, 1370, 99–111.
Fan, Y. S., et al. (2019). Impaired interactions among white-matter functional networks in antipsychotic-naive first-episode schizophrenia. Human Brain Mapping, 41(1), 230–240.
Focke, N. K., et al. (2008). Voxel-based diffusion tensor imaging in patients with mesial temporal lobe epilepsy and hippocampal sclerosis. NeuroImage, 40(2), 728–737.
Gawryluk, J. R., Mazerolle, E. L., & D’Arcy, R. C. N. (2014). Does functional MRI detect activation in white matter? A review of emerging evidence, issues, and future directions. Frontiers in Neuroscience. https://doi.org/10.3389/fnins.2014.00239
He, H., et al. (2019). Reduction in gray matter of cerebellum in schizophrenia and its influence on static and dynamic connectivity. Human Brain Mapping, 40(2), 517–528.
Ji, G. J., et al. (2019). Regional and network properties of white matter function in Parkinson’s disease. Human Brain Mapping, 40(4), 1253–1263.
Jia, X., et al. (2019). Reconfiguration of dynamic large-scale brain network functional connectivity in generalized tonic-clonic seizures. Human Brain Mapping, 41(1), 67–79.
Jiang, Y., et al. (2019a). Aberrant prefrontal-thalamic-cerebellar circuit in schizophrenia and depression: Evidence from a possible causal connectivity. International Journal of Neural Systems, 29(5), 1850032.
Jiang, S., et al. (2018). Aberrant thalamocortical connectivity in juvenile myoclonic epilepsy. International Journal of Neural Systems, 28(1), 1750034.
Jiang, Y. C., et al. (2019b). White-matter functional networks changes in patients with schizophrenia. NeuroImage, 190, 172–181.
Jiang, Y. C., et al. (2019c). Dysfunctional white-matter networks in medicated and unmedicated benign epilepsy with centrotemporal spikes. Human Brain Mapping, 40(10), 3113–3124.
Kelly, C., et al. (2012). A convergent functional architecture of the insula emerges across imaging modalities. NeuroImage, 61(4), 1129–1142.
Klugah-Brown, B., et al. (2019a). Altered dynamic functional network connectivity in frontal lobe epilepsy. Brain Topography, 32(3), 394–404.
Klugah-Brown, B., et al. (2019b). Altered structural and causal connectivity in frontal lobe epilepsy. Bmc Neurology. https://doi.org/10.1186/s12883-019-1300-z
Kobayashi, E., et al. (2006). Temporal and extratemporal BOLD responses to temporal lobe interictal spikes. Epilepsia, 47(2), 343–354.
Kros, L., et al. (2015a). Controlling cerebellar output to treat refractory epilepsy. Trends in Neurosciences, 38(12), 787–799.
Kros, L., et al. (2015b). Cerebellar output controls generalized spike-and-wave discharge occurrence. Annals of Neurology, 77(6), 1027–1049.
Lange, T., et al. (2004). Stability-based validation of clustering solutions. Neural Computation, 16(6), 1299–1323.
Li, W., et al. (2019a). Different patterns of white matter changes after successful surgery of mesial temporal lobe epilepsy. Neuroimage: Clinical, 21, 101631.
Li, J., et al. (2019b). Exploring the functional connectome in white matter. Human Brain Mapping, 40(15), 4331–4344.
Li, H., et al. (2017). Reorganization of anterior and posterior hippocampal networks associated with memory performance in mesial temporal lobe epilepsy. Clinical Neurophysiology, 128(5), 830–838.
Lorio, S., et al. (2016). New tissue priors for improved automated classification of subcortical brain structures on MRI. NeuroImage, 130, 157–166.
Luo, C., et al. (2012). Disrupted functional brain connectivity in partial epilepsy: A resting-state fMRI study. PLoS ONE, 7(1), e28196.
Marussich, L., et al. (2017). Mapping white-matter functional organization at rest and during naturalistic visual perception. NeuroImage, 146, 1128–1141.
McDonald, C. R., et al. (2008). Subcortical and cerebellar atrophy in mesial temporal lobe epilepsy revealed by automatic segmentation. Epilepsy Research, 79(2–3), 130–138.
McIntyre, D. C., & Gilby, K. L. (2008). Mapping seizure pathways in the temporal lobe. Epilepsia, 49(Suppl 3), 23–30.
Morgan, V. L., Abou-Khalil, B., & Rogers, B. P. (2015). Evolution of functional connectivity of brain networks and their dynamic interaction in temporal lobe epilepsy. Brain Connect, 5(1), 35–44.
Morgan, V. L., et al. (2019). Divergent network properties that predict early surgical failure versus late recurrence in temporal lobe epilepsy. Journal of Neurosurgery, 132(5), 1324–1333.
Peer, M., et al. (2017). Evidence for functional networks within the human brain’s white matter. Journal of Neuroscience, 37(27), 6394–6407.
Qin, Y., et al. (2019). BOLD-fMRI activity informed by network variation of scalp EEG in juvenile myoclonic epilepsy. NeuroImage: Clinical, 22, 101759.
Rodrigo, S., et al. (2007). Uncinate fasciculus fiber tracking in mesial temporal lobe epilepsy. Initial findings. European Radiology, 17(7), 1663–1668.
Schuck, N. W., et al. (2016). Human orbitofrontal cortex represents a cognitive map of state space. Neuron, 91(6), 1402–1412.
Tellez-Zenteno, J. F., & Hernandez-Ronquillo, L. (2012). A review of the epidemiology of temporal lobe epilepsy. Epilepsy Research and Treatment, 2012, 630853.
Tzourio-Mazoyer, N., et al. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage, 15(1), 273–289.
Umeoka, S. C., et al. (2012). Requirement of longitudinal synchrony of epileptiform discharges in the hippocampus for seizure generation: A pilot study. Journal of Neurosurgery, 116(3), 513–524.
Voets, N. L., et al. (2012). Structural substrates for resting network disruption in temporal lobe epilepsy. Brain, 135, 2350–2357.
Wei, W., et al. (2016). more severe extratemporal damages in mesial temporal lobe epilepsy with hippocampal sclerosis than that with other lesions: A multimodality MRI study. Medicine, 95(10), e3020.
Wu, X., et al. (2017). Functional connectivity and activity of white matter in somatosensory pathways under tactile stimulations. NeuroImage, 152, 371–380.
Xu, S. W., et al. (2018). Cognitive decline and white matter changes in mesial temporal lobe epilepsy. Medicine (Baltimore), 97(33), e11803.
Yeo, B. T., et al. (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology, 106(3), 1125–1165.
Zhou, X., et al. (2019). Disruption and lateralization of cerebellar-cerebral functional networks in right temporal lobe epilepsy: A resting-state fMRI study. Epilepsy & Behavior, 96, 80–86.
Zhu, X., et al. (2018). Altered spontaneous brain activity in MRI-negative refractory temporal lobe epilepsy patients with major depressive disorder: A resting-state fMRI study. Journal of the Neurological Sciences, 386, 29–35.
Acknowledgements
This study was funded by grants from the National Nature Science Foundation of China (Grant Number: 61933003, 81771402, 81701076, 81771822, 81960249, 81861128001), and the ‘111’ Project (B12027). We gratefully acknowledge the participation of the study subjects and investigators.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, X., Jiang, Y., Li, W. et al. Disrupted functional connectivity in white matter resting-state networks in unilateral temporal lobe epilepsy. Brain Imaging and Behavior 16, 324–335 (2022). https://doi.org/10.1007/s11682-021-00506-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-021-00506-8