Abstract
Wilson’s disease (WD) is an inherited autosomal recessive disorder of copper metabolism, and its neurological and neuropsychiatric manifestations are associated with copper accumulation in brain. A few neuroimaging studies have shown that gray matter atrophy in WD affects both subcortical structures and cortex. This study aims to quantitatively evaluate the morphometric brain abnormalities in patients with WD in terms of whole brain volume and cortical thickness and their associations with clinical severity of WD. Thirty patients clinically diagnosed as WD with neurological manifestations and 25 healthy controls (HC) were recruited. 3D T1-weighted images were segmented into 276 whole-brain regions of interest (ROIs) and 68 cortical ROIs. WD-vs-HC group comparisons were then conducted for each ROI. The associations between those morphometric measurements and the Global Assessment Scale (GAS) score for WD were analyzed. Compared with HC, significant WD-related volumetric decreases were found in the bilateral subcortical nuclei (putamen, globus pallidus, caudate nucleus, substantia nigra, red nucleus and thalamus), diffuse white matter and several gray matter regions. WD patients showed reduced cortical thickness in the left precentral gyrus and the left insula. Further, the volumes of the right globus pallidus, bilateral putamen, right external capsule and left superior longitudinal fasciculus were negatively correlated with GAS. Our results indicated that significant WD-related morphometric abnormalities were quantified in terms of whole-brain volumes and cortical thicknesses, some of which correlated significantly to the clinical severity of WD. Those morphometrics may provide a potentially effective biomarker of WD.



Similar content being viewed by others
References
Aggarwal, A., Aggarwal, N., Nagral, A., Jankharia, G., & Bhatt, M. (2009). A novel global assessment scale for Wilson's disease (GAS for WD). Movement Disorders, 24(4), 509–518.
Ala, A., Walker, A. P., Ashkan, K., Dooley, J. S., & Schilsky, M. L. (2007). Wilson's disease. LANCET, 369(9559), 397–408.
Ashburner, J. (2009). Computational anatomy with the SPM software. Magnetic Resonance Imaging, 27(8), 1163–1174.
Bandmann, O., Weiss, K. H., & Kaler, S. G. (2015). Wilson's disease and other neurological copper disorders. The Lancet Neurology, 14(1), 103–113.
Bostan, A. C., & Strick, P. L. (2018). The basal ganglia and the cerebellum: Nodes in an integrated network. Nature Reviews Neuroscience, 19(6), 338–350.
Caruana, F., Gerbella, M., Avanzini, P., Gozzo, F., Pelliccia, V., Mai, R., Abdollahi, R. O., Cardinale, F., Sartori, I., Lo Russo, G., & Rizzolatti, G. (2018). Motor and emotional behaviours elicited by electrical stimulation of the human cingulate cortex. Brain, 141(10), 3035–3051.
Chen, B., Wang, S., Sun, W., Shang, X., Liu, H., Liu, G., Gao, J., et al. (2017). Functional and structural changes in gray matter of parkinson's disease patients with mild cognitive impairment. European Journal of Radiology 93(16-23).
Członkowska, A., Litwin, T., Dusek, P., Ferenci, P., Lutsenko, S., Medici, V., Rybakowski, J. K., Weiss, K. H., & Schilsky, M. L. (2018). Wilson disease. Nature Reviews. Disease Primers, 4(1), 21.
Dale, A. M., Fischl, B., & Sereno, M. I. (1999). Cortical surface-based analysis. Neuroimage, 9(2), 179–194.
Desikan, R. S., Ségonne, F., Fischl, B., Quinn, B. T., Dickerson, B. C., Blacker, D., Buckner, R. L., Dale, A. M., Maguire, R. P., Hyman, B. T., Albert, M. S., & Killiany, R. J. (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage, 31(3), 968–980.
Ferenci, P., Caca, K., Loudianos, G., Mieli-Vergani, G., Tanner, S., Sternlieb, I., Schilsky, M., Cox, D., & Berr, F. (2003). Diagnosis and phenotypic classification of Wilson disease. Liver International, 23(3), 139–142.
Fischl, B., & Dale, A. M. (2000). Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences, 97(20), 11050–11055.
Fischl, B., Sereno, M. I., Tootell, R. B., & Dale, A. M. (1999). High-resolution intersubject averaging and a coordinate system for the cortical surface. Human Brain Mapping, 8(4), 272–284.
Fischl, B., van der Kouwe, A., Destrieux, C., Halgren, E., Segonne, F., Salat, D. H., Busa, E., et al. (2004). Automatically parcellating the human cerebral cortex. Cerebral Cortex, 14(1), 11–22.
Guo, F., Xi, Y., Gao, M., Liu, L., Fei, N., Qin, W., Li, C., et al. (2018). Alterations in cortical thickness in nonmedicated premature ejaculation patients: A morphometric MRI study. Journal of Magnetic Resonance Imaging, 47(3), 656–662.
Hamilton, M. (1959). The assessment of anxiety states by rating. The British Journal of Medical Psychology, 32(1), 50–55.
Hamilton, M. (1960). A rating scale for depression. J Neurol Neurosurg Psychiatry 23(56-62).
Hu, X., Chen, S., Huang, C., Qian, Y., & Yu, Y. (2017). Frequency-dependent changes in the amplitude of low-frequency fluctuations in patients with Wilson’s disease: A resting-state fMRI study. Metabolic Brain Disease, 32(3), 685–692.
Jadav, R., Saini, J., Sinha, S., Bagepally, B., Rao, S., & Taly, A. B. (2013). Diffusion tensor imaging (DTI) and its clinical correlates in drug naïve Wilson’s disease. Metabolic Brain Disease, 28(3), 455–462.
Kalita, J., Naik, S., Bhoi, S. K., Misra, U. K., Ranjan, A., & Kumar, S. (2017). Pontomesencephalic atrophy and postural instability in Wilson disease. American Journal of Neuroradiology, 38(7), 1343–1347.
Kamagata, K., Zalesky, A., Hatano, T., Ueda, R., Di Biase, M.A., Okuzumi, A., Shimoji, K., et al. (2017). Gray matter abnormalities in idiopathic Parkinson's disease: Evaluation by diffusional kurtosis imaging and Neurite orientation dispersion and density imaging. Human Brain Mapping.
Li, W., Qin, W., Liu, H., Fan, L., Wang, J., Jiang, T., Yu, C. (2013). Subregions of the human superior frontal gyrus and their connections. Neuroimage 78(46-58).
Li, X., Feng, Z., Tang, W., Yu, X., Qian, Y., Liu, B., Li, X., et al. (2018). Sex differences in clinical characteristics and brain MRI change in patients with Wilson's disease in a Chinese population. Frontiers in Physiology 9(1429).
Liu, C.F., Padhy, S., Ramachandran, S., Wang, V.X., Efimov, A., Bernal, A., Shi, L., et al. (2019). Using deep Siamese neural networks for detection of brain asymmetries associated with Alzheimer's disease and mild cognitive impairment. Magnetic Resonance Imaging 64(190-199).
Lorincz, M.T. (2018). Wilson disease and related copper disorders. Handb Clin Neurol 147(279-292).
Mori, S., Wu, D., Ceritoglu, C., Li, Y., Kolasny, A., Vaillant, M. A., Faria, A. V., Oishi, K., & Miller, M. I. (2016). MRICloud: Delivering high-throughput MRI Neuroinformatics as cloud-based software as a service. Computing in Science & Engineering, 18(5), 21–35.
Rodriguez-Castro, K. I. (2015). Wilson’s disease: A review of what we have learned. World Journal of Hepatology, 7(29), 2859–2870.
Stezin, A., George, L., Jhunjhunwala, K., Lenka, A., Saini, J., Netravathi, M., Yadav, R., et al. (2016). Exploring cortical atrophy and its clinical and biochemical correlates in Wilson’s disease using voxel based morphometry. Parkinsonism & Related Disorders 30(52-57).
Tang, X., Oishi, K., Faria, A. V., Hillis, A. E., Albert, M. S., Mori, S., & Miller, M. I. (2013). Bayesian parameter estimation and segmentation in the multi-atlas random orbit model. PLoS One, 8(6), e65591.
Tang, X., Holland, D., Dale, A. M., Younes, L., & Miller, M. I. (2014). Shape abnormalities of subcortical and ventricular structures in mild cognitive impairment and Alzheimer's disease: Detecting, quantifying, and predicting. Human Brain Mapping, 35(8), 3701–3725.
Tombaugh, T. N., & McIntyre, N. J. (1992). The mini-mental state examination: A comprehensive review. Journal of the American Geriatrics Society, 40(9), 922–935.
Wang, H., Pouch, A., Takabe, M., Jackson, B., Gorman, J., Gorman, R., Yushkevich, P.A. (2013). Multi-atlas segmentation with robust label transfer and label fusion. Inf Process Med Imaging 23(548-559).
Zhang, Y., Zhou, W., Wang, S., Zhou, Q., Wang, H., Zhang, B., Huang, J., Hong, B., & Wang, X. (2019). The roles of subdivisions of human insula in emotion perception and auditory processing. Cerebral Cortex, 29(2), 517–528.
Zou, L., Song, Y., Zhou, X., Chu, J., & Tang, X. (2019). Regional morphometric abnormalities and clinical relevance in Wilson's disease. Movement Disorders, 34(4), 545–554.
Acknowledgements
We thank Zhuoyi Peng, Xinbei Li, Yuliang Wang, and Yisu Tian for their research assistance, and all participants involved in this study.
Funding
This work was supported by the National Key R&D Program of China (2017YFC0112404), the National Natural Science Foundation of China (NSFC 81501546 and NSFC 81201074), and the Science and Technology Program of Guangdong Province (201508020121) and the Natural Science Foundation of Guangdong Province (2017A030313676).
Author information
Authors and Affiliations
Contributions
Conception and study design (SYK, CJP and TXY), data collection or acquisition (SYK, ZL, HXQ, QHS and ZJ), statistical analysis (SYK, ZL), interpretation of results (SYK, ZL, HYQ, QHS, TXY and CJP), drafting the manuscript work or revising it critically for important intellectual content (SYK, ZL, TXY and CJP) and approval of final version to be published and agreement to be accountable for the integrity and accuracy of all aspects of the work (All authors).
Corresponding authors
Ethics declarations
Conflict of interests
None of the authors have a conflict of interest to declare.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendices
Subjects
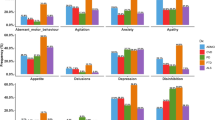
There were significant group differences in HAMD score (WD: 10.90 ± 5.71, HC: 1.88 ± 2.20) and HAM-A score (WD: 13.93 ± 7.35, HC: 1.32 ± 1.70) between WD and HC (P < 0.05). No significant differences were found in gender (P = 0.608, χ2 test), age (P = 0.900, Student’s t test), years of education (P = 0.270, Student’s t test) nor MMSE score (P = 0.133 for Student’s t test, P = 0.135 for χ2 test) between WD and HC. For the patients with WD, the mean total disease duration time was 43.87 ± 19.51 months (range: 12–84 months) and the mean GAS score was 15.64 ± 6.64 (range: 5–30). A higher GAS score indicates a more severe clinical symptom. (Table 1)
Rights and permissions
About this article
Cite this article
Song, Y., Zou, L., Zhao, J. et al. Whole brain volume and cortical thickness abnormalities in Wilson’s disease: a clinical correlation study. Brain Imaging and Behavior 15, 1778–1787 (2021). https://doi.org/10.1007/s11682-020-00373-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-020-00373-9