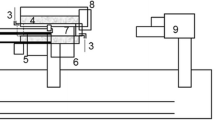
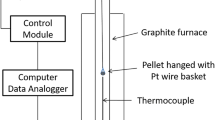
Abstract
During induration of magnetite pellets, oxidation of magnetite followed by sintering of the oxidized magnetite (hematite) is desirable. Sintering of magnetite which hampers the oxidation of magnetite is aimed to be kept as low as possible. In succession to our earlier study on sintering behavior of oxidized magnetite (hematite), this paper focusses on the sintering behavior of magnetite phase in isolation with an objective to estimate their kinetic parameters. The pellets prepared from the concentrate of LKAB’s mine, which majorly contains (>95 pct) magnetite, are used for the sintering studies. Optical Dilatometer is used to capture the sintering behavior of the magnetite pellet and determine their isothermal kinetics by deducing the three parameters, namely—activation energy (Q), pre-exponential factor (K′), and time exponent (n) with the help of power law and Arrhenius equation. It is interesting to find that the time exponent (n) is decreasing with the increase in sintering temperature. It is also interesting to note that the activation energy for sintering of magnetite pellet shows no single value. From the present investigation, two activation energies—477 kJ/mole [1173 K to 1373 K (900 °C to 1100 °C)] and 148 kJ/mole [1373 K to 1623 K (1100 °C to 1350 °C)]—were deduced for sintering of magnetite, suggesting two different mechanisms operating at lower and other at higher temperatures. The estimated kinetic parameters were used to predict the non-isothermal sintering behavior of magnetite using the sintering kinetic model. Predicted results were validated using experimental data.














Similar content being viewed by others
Abbreviations
- δ A,overall :
-
Overall percentage area change at any instant during induration
- δ A,sintering :
-
Percentage area change due to sintering at any instant during induration
- δ A :
-
Percentage area change at any instant
- δ A,true :
-
Percentage area change when pellet has no pores
- α :
-
Volumetric thermal coefficient of expansion
- β :
-
Area thermal coefficient of expansion
- V 0 :
-
Initial volume of a material
- V :
-
Volume of material at any temperature
- V true :
-
Volume of the pellet if it would have undergone complete sintering with no pores
- T 0 :
-
Initial temperature (t = 0)
- T :
-
Temperature at any instant
- γ :
-
Sintering Ratio of the pellet at any instant in the isothermal section
- γ*:
-
Sintering ratio at the start of the isothermal section
- ρ :
-
Bulk density of the pellet at any instant
- ρ true :
-
True density of the pellet
- ρ 0 :
-
Initial bulk density of pellet
- t :
-
Time for sintering reaction
- t*:
-
Time corresponds if the pellet had attained a sintering ratio of γ * from the start under isothermal condition
- t m :
-
Measured time in isothermal section
- n :
-
Time exponent
- \( K_{1}^{\prime } \) :
-
Pre-exponential factor at high temperatures
- \( K_{2}^{\prime } \) :
-
Pre-exponential factor at low temperatures
- Q 1 :
-
Activation energy at high temperatures
- Q 2 :
-
Activation energy at low temperatures
- R :
-
Universal gas constant
- γ t :
-
Sintering ratio at time t
- γ t+∆t :
-
Sintering ratio at time t + ∆t
- Tt :
-
Temperature at time t
- T t+∆t :
-
Temperature at time t + ∆t
- K(T t+∆t ):
-
Rate constant at t + ∆t
References
S. P. E. Forsmo, S. -. Forsmo, P. -. Samskog, and B. M. T. Björkman: Powder Technol, 2008, vol. 183, pp. 247-59.
S. R. Cooke and W. F. Stowasser Jr: Trans. AIME, 1952, vol. 193, pp. 1223-30.
V. Niiniskorpi: Ironmaking Conference Proceedings, 2001, pp. 767–80.
M. Tang, H.J. Cho, and P.C. Pistorius: Metall. Mater. Trans. B, 2014, vol. 45B, pp. 1304–14.
J. R. Wynnyckyj and W. A. McCurdy: Metall Trans., 1974, vol. 5, pp. 2207-15.
P. Semberg, A. Rutqvist, C. Andersson, and B. Björkman: Ironmaking and Steelmaking, 2011, vol. 38, pp. 321–28.
P. Semberg, C. Andersson, and B. Björkman: ISIJ Int., 2013, vol. 53, pp. 391-98.
J. Thurlby, R. Batterham, and R. Turner: Int. J. Miner. Process., 1979, vol. 6, pp. 43-64.
J. Thurlby: Metall. Trans. B, 1988, vol. 19B, pp. 103-12.
R. A. Dave: Can. J. Chem. Eng., 1996, vol. 74, pp. 1004-09.
M. Cross and P. Blot: Metall. and Mater. Trans. B, 1999, vol. 30B, pp. 803-13.
S. Sadrnezhaad, A. Ferdowsi, and H. Payab: Comput. Mater. Sci., 2008, vol. 44, pp. 296-02.
M. Barati: Int. J. Miner. Process., 2008, vol. 89, pp. 30-39.
T.K. SandeepKumar, N.N. Viswanathan, H.M. Ahmed, C. Andersson, B. Björkman: Metall. Mater. Trans. B, 2015, vol. 46B, pp. 635-43.
G. C. Kuczynski: Journal of Applied Physics, 1949, vol. 20, pp. 1160-63.
D. L. Johnson and I. B. Cutler: J. Am. Ceram. Soc., 1963, vol. 46, pp. 545-50.
W. S. Young and I. B. Cutler: J. Am. Ceram. Soc., 1970, vol. 53, pp. 659-63.
J. D. Hansen, R. P. Rusin, M. Teng, and D. L. Johnson: J. Am. Ceram. Soc., 1992, vol. 75, pp. 1129-35.
M. V. Nikolić, V. P. Pavlović, V. B. Pavlović, N. Labus, and B. Stojanović: Mater. Sci. Forum, 2005, vol. 494, pp. 417-22.
P. Dehaudt, L. Bourgeois, and H. Chevrel: J. Nucl. Mater., 2001, vol. 299, pp. 250-59.
W. Zeng, L. Gao, L. Gui, and J. Guo: Ceram. Int., 1999, vol. 25, pp. 723-26.
Z. Holková, L. Pach, V. Kovár, and S. Svetík: Ceramics-Silik., 2003, vol. 47, pp. 13-19.
T. Kutty, K. Khan, P. Hegde, A. Sengupta, S. Majumdar, and H. Kamath: Sci. Sinter., 2003, vol. 35, pp. 125-32.
D. Lahiri, S. Rao, G. Rao, and R. Srivastava: J. Nucl. Mater., 2006, vol. 357, pp. 88-96.
M. Mazaheri, F. Golestani-Fard, and A. Simchi: 10th International Conference and Exhibition of the European Ceramic Society, Berlin, 2007.
Z. Y. Liu, N. H. Loh, K. A. Khor, and S. B. Tor: Scr. Mater., 2001, vol. 44, pp. 1131-37.
R. L. Coble: J. Am. Ceram. Soc., 1958, vol. 41, pp. 55-62.
J. R. Wynnyckyj and T. Z. Fahidy: Metall. Trans., 1974, vol. 5, pp. 991-1000.
A. Karamanov, B. Dzhantov, M. Paganelli, and D. Sighinolfi: Thermochim. Acta, 2013, vol. 553, pp. 1-7.
V. N. Nurni and B. N. Ballal: Treatise in Process Metallurgy, vol. 1, Elsevier, Oxford, UK, 2014.
S.R.B. Cooke and T. E. Ban: Transactions AIME Min Eng., 1952, vol. 6, pp. 1053-58.
D. R. Gaskell: Introduction to the Thermodynamics of Materials, 2nd ed., CRC Press, New York, USA, 2008, pp. 6.
B. J. Skinner: Thermal Expansion - Geological Society of America Memoirs, 1966, vol. 97, pp. 75-96.
D. Taylor: Transactions and journal of the British Ceramic Society, 1985, vol. 84, pp. 121-127.
G. C. Kuczynski: AIME Trans., 1949, vol. 185, pp. 169-78.
R. German and Z. Munir: Metallurgical and Materials Transactions A, 1975, vol. 6, pp. 2229-34.
R. M. German: Sintering Theory and Practice, Wiley Interscience, New York, 1996.
R. German and Z. Munir: J. Am. Ceram. Soc., 1976, vol. 59, pp. 379-83.
R. German and Z. Munir: J. Mater. Sci., 1975, vol. 10, pp. 1719-24.
F. Nichols: Acta Metall., 1968, vol. 16, pp. 103-13.
W. Beere: Journal of Materials Science, 1973, vol. 8, pp. 1717-24.
W. Kingery: J. Appl. Phys., 1960, vol. 31, pp. 833-38.
J. Dedrick and A. Gerds: J. Appl. Phys., 1949, vol. 20, pp. 1042-44.
Z. Xuebin, D. Yunfei, W. Songlin, X. Jie, F. Yi: Ceram. Silik., 2010, vol. 54, pp. 248-252.
R. Dieckmann and H. Schmalzried: Berichte Der Bunsengesellschaft Für Physikalische Chemie, 1977, vol. 81, pp. 344-47.
R. Dieckmann and H. Schmalzried: Berichte Der Bunsengesellschaft Für Physikalische Chemie, 1977, vol. 81, pp. 414-19.
G. Lewis, C. R. A. Catlow, and A. Cormack: Journal of Physics and Chemistry of Solids, 1985, vol. 46, pp. 1227-33.
S. Hallström, L. Höglund, and J. Ågren: Acta Materialia, 2011, vol. 59, pp. 53-60.
H. Schmalzried: Reactivity of Solids, 1988, vol. 5, pp. 269-78.
N. L. Peterson, W. Chen, and D. Wolf: Journal of Physics and Chemistry of Solids, 1980, vol. 41, pp. 709-19.
J. A. Van Orman and K. L. Crispin: Reviews in Mineralogy and Geochemistry, 2010, vol. 72, pp. 757-825.
D. Levy, R. Giustetto, and A. Hoser: Physics and Chemistry of Minerals, 2012, vol. 39, pp. 169-76.
K. D. Becker, V. V. Wurmb, and F. J. Litterst: Journal of Physics and Chemistry of Solids, 1993, vol. 54, pp. 923-35.
K. D. Becker, V. V. Wurmb, and F. J. Litterst: Hyperfine Interactions, 1990, vol. 56, pp. 1431-35.
K. Becker: Solid State Ionics, 1990, vol. 39, pp. 27-35.
F. Litterst: Hyperfine Interactions, 1986, vol. 27, pp. 123-34.
L. Bentell: Scandinivian Journal of Metallurgy, 1981, vol. 10, pp. 205-09.
Acknowledgments
The authors thank the Hjalmar Lundbohm Research Centre (HLRC) for their financial support. We also thank Ola Eriksson, Daniel Marjavaara, Gustaf Magnusson, Magnus Stafstedt, and Anders Dahlin of LKAB for their technical support. We also thank Prof. S. Seetharaman, an Emeritus professor of Royal Institute of Technology (KTH), Stockholm for valuable discussions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted June 3, 2015
An erratum to this article is available at http://dx.doi.org/10.1007/s11663-016-0843-2.
Rights and permissions
About this article
Cite this article
Sandeep Kumar, T.K., Viswanathan, N.N., Ahmed, H.M. et al. Estimation of Sintering Kinetics of Magnetite Pellet Using Optical Dilatometer. Metall Mater Trans B 47, 309–319 (2016). https://doi.org/10.1007/s11663-015-0505-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-015-0505-9