Abstract
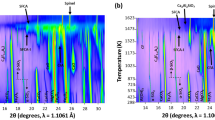
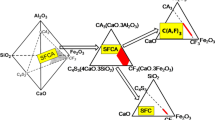
Effects of basicity, B (CaO:SiO2 ratio) on the thermal range, concentration, and formation mechanisms of silico-ferrite of calcium and aluminum (SFCA) and SFCA-I iron ore sinter bonding phases have been investigated using an in situ synchrotron X-ray diffraction-based methodology with subsequent Rietveld refinement-based quantitative phase analysis. SFCA and SFCA-I phases are the key bonding materials in iron ore sinter, and improved understanding of the effects of processing parameters such as basicity on their formation and decomposition may assist in improving efficiency of industrial iron ore sintering operations. Increasing basicity significantly increased the thermal range of SFCA-I, from 1363 K to 1533 K (1090 °C to 1260 °C) for a mixture with B = 2.48, to ~1339 K to 1535 K (1066 °C to 1262 °C) for a mixture with B = 3.96, and to ~1323 K to 1593 K (1050 °C to 1320 °C) at B = 4.94. Increasing basicity also increased the amount of SFCA-I formed, from 18 wt pct for the mixture with B = 2.48 to 25 wt pct for the B = 4.94 mixture. Higher basicity of the starting sinter mixture will, therefore, increase the amount of SFCA-I, considered to be more desirable of the two phases. Basicity did not appear to significantly influence the formation mechanism of SFCA-I. It did, however, affect the formation mechanism of SFCA, with the decomposition of SFCA-I coinciding with the formation of a significant amount of additional SFCA in the B = 2.48 and 3.96 mixtures but only a minor amount in the highest basicity mixture. In situ neutron diffraction enabled characterization of the behavior of magnetite after melting of SFCA produced a magnetite plus melt phase assemblage.







Similar content being viewed by others
References
I. Tonžetć, and A. Dippenaar: Miner. Eng., 2011, vol. 24, pp. 1258-1263.
M. Sasaki and Y. Hida: Tetsu-To-Hagane, 1982, vol. 68, pp. 563-71.
N.A.S. Webster, M.I. Pownceby, I.C. Madsen, and J.A. Kimpton: Metall. Mater. Trans. B, 2012, vol. 43, pp. 1344-1357.
W.G. Mumme, J.M.F. Clout, and R.W. Gable: Neues Jahrb. Miner. Abh., 1998, vol. 173, pp. 93-117.
J. Hancart, V. Leroy, and A. Bragard: C.N.R.M. Report, 1967, DS 24/67, pp. 3–7.
S.N. Ashan, T. Mukkerjee, and J.A. Whiteman: Ironmak. Steelmak., 2003, vol. 10, pp. 54-64.
J. Ostwald: BHP Tech. Bull., 1981, vol. 25, pp. 13–20.
J. McAndrew and J.M.F. Clout: Proc. 4th China-Australia Symp. Technol. Feed Prep. Ironmak. Dampier, Australia, 1993, pp. 1–15.
T.R.C. Patrick and M.I. Pownceby: Metall. Mater. Trans. B, 2001, vol. 32, pp. 1-11.
N.V.Y. Scarlett, M.I. Pownceby, I.C. Madsen, and A. Christensen: Metall. Mater. Trans. B, 2004, vol. 35, pp. 929-36.
N.V.Y. Scarlett, I.C. Madsen, M.I. Pownceby, and A. Christensen: J. Appl. Cryst., 2004, vol. 37, pp. 362-68.
N.A.S. Webster, M.I. Pownceby, I.C. Madsen, and J.A. Kimpton: ISIJ Int., 2013, vol. 53, pp. 774-781.
N.A.S. Webster, M.I. Pownceby, and I.C. Madsen: ISIJ Int., 2013, vol. 53, pp. 1334-1340.
J.D.G. Hamilton, B.F. Hoskins, W.G. Mumme, W.E. Borbidge, and M.A. Montague, Neues Jahrb. Miner. Abh., 1989, vol. 161, pp. 1-26.
F. Matsuno: T. Iron Steel I. Japan, 1979, vol. 19, pp. 595-604.
F. Matsuno and T. Harada: T. Iron Steel I. Japan, 1981, vol. 21, pp. 318-25.
M.I. Pownceby and J.M.F. Clout: T. I. Min. Metall. C, 2000, vol. 109, pp. 36-48.
E. Mazanek and S. Jasieńska: J. Iron Steel I., 1968, vol. 206, pp. 1104-1109.
T. Ya Malysheva, Yu. S. Yusfin, N.R. Mansurova, M.F. Gibadulin, and V.P. Lekin: Steel Transl., 2007, vol. 37, pp. 126–130.
F. Zhang, S-.L. An, G-.P. Luo, and Y-.C. Wang: J. Iron Steel Res. Int., 2012, vol. 19, pp. 1-5.
K. Wallwork, B. Kennedy, and D. Wang: AIP Conference Proceedings, 2007, vol. 879, 879-82.
L-H. Hsieh and J.A. Whiteman: ISIJ Int., 1989, vol. 29, pp. 24-32.
Bruker, TOPAS Version 4.2, Bruker AXS Inc., Madison, WI, 2009.
R. Blake, R. Hessevick, T. Zoltai, and L. Finger: Am. Mineral., 1966, vol. 51, pp. 123-29.
E.N. Maslen, V.A. Streltsov, N.R. Streltsova, and N. Ishizawa: Acta Cryst. B, 1995, vol. 51, pp. 929–939.
H. Saalfeld and M. Wedde: Z. Krystallogr. Krist., 1974, vol. 139, pp. 129-135.
G.A. Lager, J.D. Jorgensen, and F.J. Rotella: J. Appl. Phys., 1982, vol. 53, pp. 6751-6756.
H. Schulz and V. Tscherry: Acta Cryst. B-Stru., 1972, vol. 28, pp. 2168-2173.
I.Z. Oftedal: Z. Phys. Chem., 1927, vol. 128, pp. 135-158.
P. Berastegui, S.-G. Eriksson, and S. Hull, Mater. Res. Bull., 1999, vol. 34, pp. 303-314.
D.F. Decker, J.S. Kasper, Acta Cryst., 1957, vol. 10, pp. 332-337.
S.J. Louisnathan: Can. Mineral., 1971, vol. 10, pp. 822-837.
W.C. Hamilton: Phys. Rev., 1958, vol. 110, pp. 1050-1057.
A.J. Studer, M.E. Hagen, and T.J. Noakes: Physica B, 2006, vol. 385-386, pp. 1013-1015.
K. Kihara, Eur. J. Mineral., 1990, vol. 2, pp. 63-77.
Acknowledgements
The Australian Nuclear Science and Technology Organisation (ANSTO) is acknowledged for financial support of this research. This research was partially undertaken on the powder diffraction beamline (10BM1) at the Australian Synchrotron, Victoria, Australia, under beamtime awards AS113/PD4160 and AS132/PD6321. The Australian Institute of Nuclear Science and Engineering (AINSE) is acknowledged for travel and accommodation support under award P2275 for beamtime on the WOMBAT neutron powder diffractometer. The authors wish to thank: Barry Halstead, Jean-Pierre Veder and Bree Morgan (CSIRO Mineral Resources Flagship) for assistance with synchrotron data collection; Scott Olsen, Stewart Pullen and Georgia Clarke (Bragg Institute, ANSTO) for their assistance with sample environment for the neutron diffraction experiment.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted November 24, 2013.
Rights and permissions
About this article
Cite this article
Webster, N.A.S., Pownceby, M.I., Madsen, I.C. et al. Fundamentals of Silico-Ferrite of Calcium and Aluminum (SFCA) and SFCA-I Iron Ore Sinter Bonding Phase Formation: Effects of CaO:SiO2 Ratio. Metall Mater Trans B 45, 2097–2105 (2014). https://doi.org/10.1007/s11663-014-0137-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-014-0137-5