Abstract
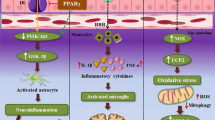
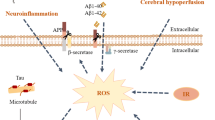
Alzheimer’s disease (AD) is the most common neurodegenerative disease characterized by progressive cognitive impairment. The pathogenesis of AD is complex, and its susceptibility and development process are affected by age, genetic and epigenetic factors. Recent studies confirmed that gut microbiota (GM) might contribute to AD through a variety of pathways including hypothalamic pituitary adrenal axis and inflflammatory and immune processes. CM formula, herbs, and monomer enjoy unique advantages to treat and prevent AD. Hence, the purpose of this review is to outline the roles of GM and its core metabolites in the pathogenesis of AD. Research progress of CMs regarding the mechanisms of how they regulate GM to improve cognitive impairment of AD is also reviewed. The authors tried to explore new therapeutic strategies to AD based on the regulation of GM using CM.
Similar content being viewed by others
References
Alzheimer’s Disease International. 2019. Attitudes to dementia. Available at: http://www.alz.co.uk/research/world-report-2019/. London: World Alzheimer Report 2019 [updated 2015 Sep].
Kang J, Lu J, Zhang X. Metabolomics-based promising candidate biomarkers and pathways in Alzheimer’s disease. Die Pharmazie 2015;70:277–282.
Forbes JD, Bernstein CN, Tremlett H, et al. A fungal world: could the gut mycobiome be involved in neurological disease? Front Microbiol 2018;9:3249.
Scirocco CM, Maselli MA, Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Annals Gastroenterol 2015;28:203–209.
Sun K, Fan J, Han J. Ameliorating effects of traditional Chinese medicine preparation, Chinese materia medica and active compounds on ischemia/reperfusion-induced cerebral microcirculatory disturbances and neuron damage. Acta Pharm Sin B 2015;5:8–24.
Harris SE, Riggio VL, Evenden T, et al. Age-related gene expression changes, and transcriptome wide association study of physical and cognitive aging traits, in the Lothian Birth Cohort 1936. Aging 2017;9:489–2503.
Villemagne VL, Masters CL. Alzheimer disease: the landscape of ageing-insights from AD imaging markers, Nature reviews. Neurology 2014;10:678–679.
Plassman BL, Langa KM, Fisher GG, et al. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology 2007;29:125–132.
Ferreira-Vieira TH, Guimaraes IM, Silva FR, et al. Alzheimer’s disease: targeting the cholinergic system. Curr Neuropharmacol 2016;14:101–115.
Bell KF, Ducatenzeiler A, Ribeiro-da-Silva A, et al. The amyloid pathology progresses in a neurotransmitter-specific manner. Neurobiol Aging 2006;27:1644–1157.
Rogers JL, Kesner RP. Cholinergic modulation of the hippocampus during encoding and retrieval of tone/shockinduced fear conditioning. Learn Memory (Cold Spring Harbor, N.Y.) 2004;11:102–107.
Hardy J, Selkoe DJ, The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 2002;297:353–356.
de JRDPV, Forlenza AS, Forlenza OV. Relevance of gutmicrobiota in cognition, behaviour and Alzheimer’s disease. Pharmacol Res 2018;136:29–34.
Garin D, Virgone-Carlotta A, Gozel B, et al. COB231 targets amyloid plaques in post-mortem human brain tissue and in an Alzheimer mouse model. J Neurochem 2015;132:609–618.
Armstrong RA, Factors determining disease duration in Alzheimer’s disease: a postmortem study of 103 cases using the Kaplan-Meier estimator and Cox regression. BioMed Res Intern 2014;2014:623487.
Klimova B, Kuca K. Alzheimer’s disease and Chinese medicine as a useful alternative intervention tool: a mini-review. Curr Alzheimer Res 2017;14:680–685.
Eikelenboom P, van Exel E, Veerhuis R, et al. Hoozemans, innate immunity and the etiology of late-onset Alzheimer’s disease. Neuro-degenerative Dis 2012;10:271–273.
Heneka MT, Kummer MP, Latz E. Innate immune activation in neurodegenerative disease, nature reviews. Immunol 2014;14:463–477.
Erickson MA, Banks WA. Blood-brain barrier dysfunction as a cause and consequence of Alzheimer’s disease. J Cerebr Blood Flow Metabol 2013;33:1500–1513.
Mancuso C, Santangelo R. Alzheimer’s disease and gut microbiota modifications: the long way between preclinical studies and clinical evidence. Pharmacol Res 2018;129:329–336.
Wu GD, Compher C, Chen EZ, et al. Comparative metabolomics in vegans and omnivores reveal constraints on diet-dependent gut microbiota metabolite production. Gut 2016;65:63–72.
Zhan G, Yang N, Li S, et al. Abnormal gut microbiota composition contributes to cognitive dysfunction in SAMP8 mice. Aging 2018;10:1257–1267.
Alkasir R, Li J, Li X, et al. Human gut microbiota: the links with dementia development. Protein Cell 2017;8:90–102.
Sherwin E, Dinan TG, Cryan JF. Recent developments in understanding the role of the gut microbiota in brain health and disease. Annals New York Academy Sci 2018;1420:5–25.
Pellegrini C, Antonioli L, Colucci R, et al. Interplay among gut microbiota, intestinal mucosal barrier and enteric neuro-immune system: a common path to neurodegenerative diseases? Acta Neuropathol 2018;136:345–361.
Bravo JA, Forsythe P, Chew MV, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A 2011;108:16050–16055.
Huffman WJ, Subramaniyan S. Modulation of neuroinflammation and memory dysfunction using percutaneous vagus nerve stimulation in mice. Brain Stimul 2019;12:19–29.
Cani PD, Knauf C. How gut microbes talk to organs: the role of endocrine and nervous routes. Mol Metab 2016;5:743–752.
Bonfili L, Cecarini V, Berardi S, et al. Microbiota modulation counteracts Alzheimer’s disease progression influencing neuronal proteolysis and gut hormones plasma levels. Sci Rep 2017;7:2426.
Qureshi IA, Mehler MF. Towards a ‘systems’-level understanding of the nervous system and its disorders. Trends Neurosci 2013;36:674e684.
Qin J, Li R, Raes J, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010;464:59–65.
Wu J, Wang Y, Zhang Z. et al. Herbal medicine in the treatment of Alzheimer’s disease. Chin J Integr Med 2015;21:102–107.
Silver R, Curley JP. Mast cells on the mind: new insights and opportunities. Trends Neurosci 2013;36:513e521.
Erny D, de Angelis ALH, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci 2015;18:965–977.
Park AM, Omura S, Fujita M, et al. Helicobacter pylori and gut microbiota in multiple sclerosis versus Alzheimer’s disease: 10 pitfalls of microbiome studies. Clin Exp Neuroimmunol 2017;8:215–232.
Harach T, Marungruang N, Duthilleul N, et al. Reduction of Abeta amyloid pathology in APPPS1 transgenic mice in the absence of gut microbiota. Sci Rep 2017;7:41802.
Weaver CT, Elson CO, Fouser LA, et al. The Th17 pathway and inflammatory diseases of the intestines, lungs, and skin. Annu Rev Pathol 2013;8:477–512.
Saksida T, Koprivica I, Vujičić M, et al. Impaired IL-17 production in gut-residing immune cells of 5xFAD mice with Alzheimer’s disease pathology. J Alzheimer’s Dis 2018;61:619–630.
Cattaneo A., Cattane N, Galluzzi S, et al. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol Aging 2017;49:60–68.
Cani PD, Amar J, Iglesias MA, et al. Metabolic ensdotoxemia initiates obesity and insulin resistance. Diabetes 2007;56:1761–1772.
Gregory JM, Livesey MR, McDade K, et al. Dysregulation of AMPA receptor subunit expression in sporadic ALS postmortem brain. J Pathol 2020;250:67–78.
Guo S, Nighot M, Al-Sadi R. Lipopolysaccharide regulation of intestinal tight junction permeability is mediated by TLR4 signal transduction pathway activation of FAK and MyD88. J Immunol 2015;195:4999–5010.
Lee HJ, Lee KE, Kim JK, et al. Suppression of gut dysbiosis by Bifidobacterium longum alleviates cognitive decline in 5XFAD transgenic and aged mice. Sci Rep 2019;9:11814.
Jang HM, Lee HJ, Jang SE, et al. Evidence for interplay among antibacterial-induced gut microbiota disturbance, neuro-inflammation, and anxiety in mice. Mucosal Immunol 2018;11:1386–1397.
Zhao Y, Dua P, Lukiw WJ. Microbial sources of amyloid and relevance to amyloidogenesis and Alzheimer’s disease (AD). J Alzheimer’s Dis Parkinsonism 2015;5:177.
Song JH, Lee JW, Shim B, et al. Glycyrrhizin alleviates neuroinflammation and memory deficit induced by systemic lipopolysaccharide treatment in mice. Molecules 2013;18:15788–15803.
Calabrese F, Rossetti AC, Racagni G, et al. Brain-derived neurotrophic factor: a bridge between inflammation and neuroplasticity. Frontiers Cellular Neurosci 2014;8:430.
Badshah H, Ali T, Shafiq-ur R, et al. Protective effect of lupeol against lipopolysaccharide-induced neuroinflammation via the p38/c-Jun N-terminal kinase pathway in the adult mouse brain. J Neuroimmune Pharmacol 2016;11:48–60.
Badshah H, Ali T, Kim MO. Osmotin attenuates LPS-induced neuroinflammation and memory impairments via the TLR4/ NFkappaB signaling pathway. Sci Rep 2016;6:24493.
Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behavior, nature reviews. Nat Rev Neurosci 2012;13:701–712.
Koh A, De Vadder F, Kovatcheva-Datchary P, et al. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell 2016;165:1332–1345.
Lei E, Vacy K, Boon WC. Fatty acids and their therapeutic potential in neurological disorders. Neurochem Intern 2016;95:75–84.
Ho L, Ono K, Tsuji M, et al. Protective roles of intestinal microbiota derived short chain fatty acids in Alzheimer’s disease-type beta-amyloid neuropathological mechanisms. Expert Rev Neurother 2018;18:83–90.
den Besten G, van Eunen K, Groen AK, et al. The role of shortchain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res 2013;54:2325–2240.
Zilberter Y, Zilberter M. The vicious circle of hypometabolism in neurodegenerative diseases: ways and mechanisms of metabolic correction. J Neurosci Res 2017;95:2217–2235.
Erny D, Hrabe de Angelis AL, Jaitin D, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci 2015;18:965–977.
Morrison DJ, Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016;7:189–200.
Manco M, Putignani L, Bottazzo GF. Gut microbiota, lipopolysaccharides, and innate immunity in the pathogenesis of obesity and cardiovascular risk. Endocr Rev 2010;31:817–844.
Welcome MO. Gut microbiota disorder, gut epithelial and blood-brain barrier dysfunctions in etiopathogenesis of dementia: molecular mechanisms and signaling pathways. Neuromolecular Med 2019;21:205–226.
Hoyles L, Snelling T, Umlai UK, et al. Microbiome-host systems interactions: protective effects of propionate upon the bloodbrain barrier. Microbiome 2018;6:55.
Zhang L, Wang Y, Xiayu X, et al. Altered gut microbiota in a mouse model of Alzheimer’s disease. J Alzheimers Dis 2017;60:1241–1257.
Westfall S, Iqbal U, Sebastian M, et al. Gut microbiota mediated allostasis prevents stress-induced neuroinflammatory risk factors of Alzheimer’s disease. Prog Molecular Bio Transl Sci 2019;168:1877–1173.
Booth T, Royle NA, Corley J, et al. Association of allostatic load with brain structure and cognitive ability in later life. Neurobiol Aging 2015;36:1390–1399.
Ross JA, Gliebus G, van Bockstaele EJ. Stress induced neural reorganization: a conceptual framework linking depression and Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry 2018;85:136–151.
Cai M, Lee JH, Yang EJ. Electroacupuncture attenuates cognition impairment via anti-neuroinflammation in an Alzheimer’s disease animal model. J Neuroinflam 2019;16:264.
Su H, Zhang C, Zou X, et al. Jiao-tai-wan inhibits inflammation of the gut-brain-axis and attenuates cognitive impairment in insomnic rats. J Ethnopharmacol 2019;250:112478.
Zhang J, Yang C, Wei D, et al. Long-term efficacy of Chinese medicine Bushen Capsule on cognition and brain activity in patients with amnestic mild cognitive impairment. Pharmacol Res 2019;146:104319.
Liu Q, Wang SC, Ding K, et al. Research advances in the treatment of Alzheimer’s disease with polysaccharides from traditional Chinese medicine. Chin J Nat Med 2017;15:641–652.
Lv M, Yang S, Cai L, et al. Effects of quercetin intervention on cognition function in APP/PS1 mice was affected by vitamin D status. Mol Nutr Food Res 2018; 62: e1800621.
Ding RR, Chen W, Guo CY, et al. Dangguishaoyao-San attenuates LPS-induced neuroinflammation via the TLRs/NF-κB signaling pathway, Biomed Pharmacother 2018;105:187–194.
Zhang RZ, Zhu X, Bai H, et als. Network pharmacology databases for traditional Chinese medicine: review and assessment. Front Pharmacol 2019;10:123.
Zhou W, Cheng X, Zhang Y, Effect of Liuwei Dihuang Decoction, a traditional Chinese medicinal prescription, on the neuroendocrine immunomodulation network. Pharmacol Therapeutics 2016;162:170–178.
Wang J, Ye F, Cheng X, et al. The effects of LW-AFC on intestinal microbiome in senescence-accelerated mouse prone 8 strain, a mouse model of Alzheimer’s disease. J Alzheimers Dis 2016;53:907–919.
Wang J, Lei X, Xie Z, et al. CA-30, an oligosaccharide fraction derived from Liuwei Dihuang Decoction, ameliorates cognitive deterioration via the intestinal microbiome in the senescence-accelerated mouse prone 8 strain. Aging 2019;11:3463–3486.
Chu H, Zhang A, Han Y, et al. Metabolomics approach to explore the effects of Kai-Xin-San on Alzheimer’s disease using UPLC/ESI-Q-TOF mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2016;1015–1016.
Fu H, Xu Z, Zhang XL, et al. Kaixinsan, a well-known Chinese herbal prescription, for Alzheimer’s disease and depression: a preclinical systematic review}. Front Neurosci 2019;13:1421.
Kang A, Xie T, Zhu D, et al. Suppressive effect of Ginsenoside Rg3 against lipopolysaccharide-induced depression-like behavior and neuroinflammation in mice. J Agric Food Chem 2017;65:6861–6869.
Wang C, Yang S, Gao L, et al. Carboxymethyl pachyman (CMP) reduces intestinal mucositis and regulates the intestinal microflora in 5-fluorouracil-treated CT26 tumour-bearing mice. Food Funct 2018 23;9:2695–2704.
Okamoto H, Chino A, Hirasaki Y, et al. Orengedoku-to augmentation in cases showing partial response to yokukan-san treatment: a case report and literature review of the evidence for use of these Kampo herbal formulae. Neuropsychiatr Dis Treat 2013;9:151–155.
Zheng Y, Cheng XR, Zhou WX, et al. Gene expression patterns of hippocampus and cerebral cortex of senescence-accelerated mouse treated with Huang-Lian-Jie-Du Decoction. Neurosci Lett 2008;439:119–124.
Yu CJ, Zheng MF, Kuang CX, et al. Oren-gedoku-to and its constituents with therapeutic potential in Alzheimer’s disease inhibit indoleamine 2,3-dioxygenase activity in vitro. J Alzheimers Dis 2010;22:257–266.
Chen M, Liao Z, Lu B, et al. Huang-Lian-Jie-Du-Decoction ameliorates hyperglycemia and insulin resistant in association with gut microbiota modulation. Front Microbiol 2018;9:2380.
Yuan ZW, Yang LH, Zhang XS, et al. Huang-Lian-Jie-Du Decoction ameliorates acute ulcerative colitis in mice via regulating NF-κB and Nrf2 signaling pathways and enhancing intestinal barrier function. Front Pharmacol 2019;10:1354.
Wang ZL, Wang S, Kuang Y, et al. A comprehensive review on phytochemistry, pharmacology, and flavonoid biosynthesis of Scutellaria baicalensis. Pharm Biol 2018;56:465–484.
Kou J, Zhu D, Yan YJ. Neuroprotective effects of the aqueous extract of the Chinese medicine Danggui-Shaoyao-san on aged mice. J Ethnopharmacol 2005;97:313–318.
Ding RR, Chen W, Guo CY,et al. Dangguishaoyao-San attenuates LPS-induced neuroinflammation via the TLRs/NF-κB signaling pathway. Biomed Pharmacotherapy 2018;9:187–194.
Wang J, Feng WW, Zhang SY, et al. Ameliorative effect of atractylodes macrocephala essential oil combined with Panax Ginseng Total Saponins on 5-fluorouracil induced diarrhea is associated with gut microbial modulation. J Ethnopharmacol 2019;238:111887.
Su J, Pan YW, Wang SQ, et al. Saikosaponin-d attenuated lipopolysaccharide-induced depressive-like behaviors via inhibiting microglia activation and neuroinflammation. Intern Immunopharmacol 2020;3:106181.
Ashour ML, Wink M. Genus bupleurum: a review of its phytochemistry, pharmacology and modes of action. J Pharm Pharmacol 2011;63:305–321.
Sun X, Shi Z, Li T, et al. Antidepressant-like effects of total saikosaponins of Bupleurum yinchowense in mice. J Med Plants Res 2012;6:4308–4316.
Zhao SS, Yang WN, Jin H, et al. Puerarin attenuates learning and memory impairments and inhibits oxidative stress in STZinduced SAD mice. Neurotoxicology 2015;51:166–171.
Liu S, Cao XL, Liu GQ, et al. The in silico and in vivo evaluation of puerarin against Alzheimer’s disease. Food Funct 2019;10:799–813.
Qiu ZK, Zhang GH, Zhong DS. Puerarin ameliorated the behavioral deficits induced by chronic stress in rats. Front Pharmacol 2018;9:967.
Fu Z, Fan X, Wang X, et al. Cistanches Herba: an overview of its chemistry, pharmacology, and pharmacokinetics property. J Ethnopharmacol 2018;219:233–247.
Li Z, Lin H, Gu L, et al. Herba Cistanche (Rou Cong-Rong): one of the best pharmaceutical gifts of traditional Chinese medicine. Front Pharmacol 2016;7:41.
Li N, Wang JP, Ma J, et al. Neuroprotective effects of Cistanches Herba therapy on patientss with moderate Alzheimer’s disease. Evid Based Complement Alternat Med 2015;2015:103985.
Li Y, Peng Y, Ma P, et al. Antidepressant-like effects of Cistanche tubulosa extract on chronic unpredictable stress rats through restoration of gut microbiota homeostasis. Front Pharmacol 2018;9:967.
Diaz-Gerevini GT, Repossi G, Dain A, et al. Beneficial action of resveratrol: how and why? Nutrition 2016;32:174–178.
Zeng P, Shi Y, Wang XM, et al. Emodin rescued hyperhomocysteinemia-induced dementia and Alzheimer’s disease-like features in rats. Int J Neuropsychopharmacol 2019;22:57–70.
Or TCT, Yang CLH, Law AHY, et al. Isolation and identification of anti-inflammatory constituents from Ligusticum chuanxiong and their underlying mechanisms of action on microglia. Neuropharmacology 2011;5:823–831.
Wang XY, Sun GQ, Feng T, et al. Sodium oligomannate therapeutically remodels gut microbiota and suppresses gut bacterial amino acids-shaped neuroinflammation to inhibit Alzheimer’s disease progression. Cell Res 2019;29:787–803.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by National Natural Science Foundation of China (No. 81704004), Scientific and Technological Research Program of Tianjin Municipal Education Commission (No. 2018KJ031), Research and Innovation Project of Graduate (No. YJSKC-20191003, No. ZXYCXLX201804)
Rights and permissions
About this article
Cite this article
Sun, Yx., Jiang, Xj., Lu, B. et al. Roles of Gut Microbiota in Pathogenesis of Alzheimer’s Disease and Therapeutic Effects of Chinese Medicine. Chin. J. Integr. Med. 28, 1048–1056 (2022). https://doi.org/10.1007/s11655-020-3274-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11655-020-3274-5