Abstract
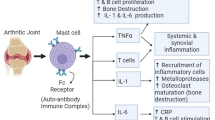
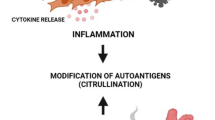
Rheumatoid arthritis (RA) is characterized as a chronic inflammatory disease in joints and concomitant destruction of cartilage and bone. Cartilage extracellular matrix components, such as type II collagen and aggrecan are enzymatically degraded by matrix metalloproteinases (MMPs) and aggrecanases in RA. Currently, treatments targeting cytokines, including anti-tumor necrosis factor (TNF) α antibodies, soluble TNF receptor, anti-interleukin (IL)-6 receptor antibody, and IL-1 receptor antagonist, are widely used for treating RA in addition to antiantiinflammatory agents and disease-modifying antirheumatic drugs (DMARDs), such as inflmethotrexate, but these treatments have some problems, especially in terms of cost and the increased susceptibility of patients to infection in addition to the existence of low-responders to these treatments. Therefore, therapeutics that can be safely used for an extended period of time would be preferable. Complementary and alternative medicines including traditional Chinese medicines (TCM) have been used for the arthritic diseases through the ages. Recently, there are many reports concerning the anti-arthritic action mechanisms of TCM-based herbal formulas and crude herbal extracts or isolated ingredients. These natural herbal medicines are thought to moderately improve RA, but they exert various actions for the treatment of RA. In this review, the current status of the mechanism exploration of natural compounds and TCM-based herbal formulas are summarized, focusing on the protection of cartilage destruction in arthritic diseases including RA and osteoarthritis.
Similar content being viewed by others
References
Scott DL, Wolfe F, Huizinga TWJ. Rheumatoid arthritis. Lancet 2010;376:1094–1108.
Goldring MB, Marcu KB. Cartilage homeostasis in health and rheumatic diseases. Arthritis Res Ther 2009;11:224.
Smolen JS, Landewé R, Breedveld FC, Dougados M, Emery P, Gaujoux-Viala C, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs. Ann Rheum Dis 2010;69:964–975.
Zhang P, Li J, Han Y, Yu XW, Qin L. Traditional Chinese medicine in the treatment of rheumatoid arthritis: a general review. Rheumatol Int 2010;30:713–718.
Venkatesha SH, Berman BM, Moudgil KD. Herbal medicinal products target defined biochemical and molecular mediators of inflammatory autoimmune arhritis. Bioorg Med Chem 2011;19:21–29.
Aigner T, Stöve J. Collagens-major component of the physiological cartilage matrix, major target of cartilage degeneration, major tool in cartilage repair. Adv Drug Deliv Rev 2003;55:1569–1593.
Roughley PJ. The structure and function of cartilage proteoglycans. Eur Cells Mater 2006;12:92–101.
Murphy G, Nagase H. Reappraising metalloproteinases in rheumatoid arthritis and osteoarthritis: destruction or repair? Nat Clin Pract Rheumatol 2008;4:128–135.
Okada Y, Gonoji Y, Nakanishi I, Nagase H, Hayakawa T. Immunohistochemical demonstration of collagenase and tissue inhibitor of metalloproteinases (TIMP) in synovial lining cells of rheumatoid synovium. Virchows Arch B Cell Pathol Incl Mol Pathol 1990;59:305–312.
Wu W, Billinghurst RC, Pidoux I, Antoniou J, Zukor D, Tanzer M, et al. Sites of collagenase cleavage and denaturation of type II collagen in aging and osteoarthritic articular cartilage and their relationship to the distribution of matrix metalloproteinase 1 1 and matrix metalloproteinase 13. Arthritis Rheum 2002;46:2087–2094.
Yoshihara Y, Nakamura H, Obata K, Yamada H, Hayakawa T, Fujikawa K, et al. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in synovial fluids from patients with rheumatoid arthritis or osteoarthritis. Ann Rheum Dis 2000;59:455–461.
Nagase H, Kashiwagi M. Aggrecanases and cartilage matrix degradation. Arthritis Res Ther 2003;5:94–103.
Sandy JD, Flannery CR, Neame PJ, Lohmander LS. The structure of aggrecan fragments in human synovial fluid. Evidence for the involvement in osteoarthritis of a novel proteinase which cleaves the Glu 373-Ala 374 bond of the interglobular domain. J Clin Invest 1992;89:1512–1516.
Lark MW, Bayne EK, Flanagan J, Harper CF, Hoerrner LA, Hutchinson NI, et al. Aggrecan degradation in human cartilage. Evidence for both matrix metalloproteinases and aggrecanase activity in normal, osteoarthritic, and rheumatoid joints. J Clin Invest 1997;100:93–106.
Struglics A, Larsson S, Pratta MA, Kumar S, Lark MW, Lohmander LS. Human osteoarthritis synovial fluid and joint cartilage contain both aggrecanase- and matrix metalloproteinase-generated aggrecan fragments. Osteoarthritis Cartilage 2006;14:101–113.
Tortorella MD, Burn TC, Pratta MA, Abbaszade I, Hollis JM, Liu R, et al. Purification and cloning of aggrecanase-1: a member of the ADAMTS family of proteins. Science 1999;284:1664–1666.
Abbaszade I, Liu RQ, Yang F, Rosenfeld SA, Ross OH, Link JR, et al. Cloning and characterization of ADAMTS11, an aggrecanase from the ADAMTS family. J Biol Chem 1999;274:23443–23450.
Stanton H, Rogerson FM, East CJ, Golub SB, Lawlor KE, Meeker CT, et al. ADAMTS5 is the major aggrecanase in mouse cartilage in vivo and in vitro. Nature 2005;434:648–652.
Glasson SS, Askew R, Sheppard B, Carito B, Blanchet T, Ma HL, et al. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature 2005;434:644–648.
Goldbach-Mansky R, Wilson M, Fleischmann R, Olsen N, Silverfield J, Kempf P, et al. Comparison of Tripterygium wilfordii Hook F versus sulfasalazine in the treatment of rheumatoid arthritis: a randomized trial. Ann Intern Med 2009;151:229–240.
Kusunoki N, Yamazaki R, Kitasato H, Beppu M, Aoki H, Kawai S. Triptolide, an active compound identified in a traditional Chinese herb, induces apoptosis of rheumatoid synovial fibroblasts. BMC Pharmacol 2004;4:2.
Wang B, Ma L, Tao X, Lipsky PE. Triptolide, an active component of the Chinese herbal remedy Tripterygium wilfordii Hook F, inhibits production of nitric oxide by decreasing inducible nitric oxide synthase gene transcription. Arthritis Rheum 2004;50:2995–3003.
Yan SX, Wang Y. Inhibitory effects of triptolide on interferon-gamma-induced human leucocyte antigen-DR, intercellular adhesion molecule-1, CD40 expression on retro-ocular fibroblasts derived from patients with Graves’ ophthalmopathy. Clin Exp Ophthalmol 2006;34:265–271.
Chan MA, Kohlmeier JE, Branden M, Jung M, Benedict SH. Triptolide is more effective in preventing T cell proliferation and interferon-gamma production than is FK506. Phytother Res 1999;13:464–467.
Lin N, Sato T, Ito A. Triptolide, a novel diterpenoid triepoxide from Tripterygium wilfordii Hook. F., suppresses the production and gene expression of pro-matrix metalloproteinases 1 and 3 and augments those of tissue inhibitors of metalloproteinases 1 and 2 in human synovial fibroblasts. Arthritis Rheum 2001;44:2193–2200.
Lin N, Chunfang L, Xiao C, Hongwei J, Imada K, Wu H, et al. Triptolide, a diterpenoid triepoxide, suppresses inflammation and cartilage destruction in collagen-induced arthritis mice. Biochem Pharmacol 2007;73:136–146.
Liacini A, Sylvester J, Zafarullah M. Triptolide suppresses proinflammatory cytokine-induced matrix metalloproteinase and aggrecanase-1 gene expression in chondrocytes. Biochem Biophys Res Commun 2005;327:320–327.
Lu Y, Fukuda K, Nakamura Y, Kimura K, Kumagai N, Nishida T. Inhibitory effect of triptolide on chemokine expression induced by proinflammatory cytokines in human corneal fibroblasts. Invest Ophthalmol Vis Sci 2005;46:2346–2352.
Ahmed S. Green tea polyphenol epigallocatechin 3-gallate in arthritis: progress and promise. Arthritis Res Ther 2010;12:208–216.
Andriamanalijaona R, Kypriotou M, Baugé C, Renard E, Legendre F, Raoudi M, et al. Comparative effects of 2 antioxidants, selenomethionine and epigallocatechin-gallate, on catabolic and anabolic gene expression of articular chondrocytes. J Rheumatol 2005;32:1958–1967.
Vankemmelbeke MN, Jones GC, Fowles C, Ilic MZ, Handley CJ, Day AJ, et al. Selective inhibition of ADAMTS-1, -4 and -5 by catechin gallate esters. Eur J Biochem 2003;270:2394–2403.
Cudic M, Burstein GD, Fields GB, Lauer-Fields J. Analysis of flavonoid-based pharmacophores that inhibit aggrecanases (ADAMTS-4 and ADAMTS-5) and matrix metalloproteinases through the use of topologically constrained peptide substrates. Chem Biol Drug Des 2009;74:473–482.
Ahmed S, Pakozdi A, Koch AE. Regulation of interleukin-1-induced chemokine production and matrix metalloproteinase 2 activation by epigallocatechin-3-gallate in rheumatoid arthritis synovial fibroblasts. Arthritis Rheum 2006;54:2393–2401.
Marotte H, Ruth JH, Campbell PL, Koch AE, Ahmed S. Green tea extract inhibits chemokine production, but up-regulates chemokine receptor expression, in rheumatoid arthritis synovial fibroblasts and rat adjuvant-induced arthritis. Rheumatology (Oxford) 2010;49:467–479.
Lee JH, Jin H, Shim HE, Kim HN, Ha H, Lee ZH. Epigallocatechin-3-gallate inhibits osteoclastogenesis by down-regulating c-Fos expression and suppressing the NF-κB signal. Mol Pharmacol 2010;77:17–25.
Kawabata K, Murakami A, Ohigashi H. Nobiletin, a citrus flavonoid, down-regulates matrix metalloproteinase-7 (matrilysin) expression in HT-29 human colorectal cancer cells. Biosci Biotechnol Biochem 2005;69:307–314.
Murakami A, Nakamura Y, Torikai K, Tanaka T, Koshiba T, Koshimizu K, et al. Inhibitory effect of citrus nobiletin on phorbol ester-induced skin inflammation, oxidative stress, and tumor promotion in mice. Cancer Res 2000;60:5059–5066.
Sato T, Koike L, Miyata Y, Hirata M, Mimaki Y, Sashida Y, et al. Inhibition of activator protein-1 binding activity and phosphatidylinositol 3-kinase pathway by nobiletin, a polymethoxy flavonoid, results in augmentation of tissue inhibitor of metalloproteinase-1 production and suppression of production of matrix metalloproteinases-1 and -9 in human fibrosarcoma HT-1080 cells. Cancer Res 2002;62:1025–1029.
Ishiwa J, Sato T, Mimaki Y, Sashida Y, Yano M, Ito A. A citrus flavonoid, nobiletin, suppresses production and gene expression of matrix metalloproteinase 9/gelatinase B in rabbit synovial fibroblasts. J Rheumatol 2000;27:20–25.
Lin N, Sato T, Takayama Y, Mimaki Y, Sashida Y, Yano M, et al. Novel anti-inflammatory actions of nobiletin, a citrus polymethoxy flavonoid, on human synovial fibroblasts and mouse macrophages. Biochem Pharmacol 2003;65:2065–2071.
Imada K, Lin N, Liu C, Lu A, Chen W, Yano M, et al. Nobiletin, a citrus polymethoxy flavonoid, suppresses gene expression and production of aggrecanases-1 and -2 in collagen-induced arthritic mice. Biochem Biophys Res Commun 2008;373:181–185.
Murakami A, Song M, Katsumata S, Uehara M, Suzuki K, Ohigashi H. Citrus nobiletin suppresses bone loss in ovariectomized ddY mice and collagen-induced arthritis in DBA/1J mice: possible involvement of receptor activator of NF-kappaB ligand (RANKL)-induced osteoclastogenesis regulation. Biofactors 2007;30:179–192.
Aggarwal BB, Sung B. Pharmacological basis for the role of curcumin in chronic diseases: an age-old spice with modern targets. Trends Pharmacol Sci 2009;30:85–94.
Bar-Sela G, Epelbaum R, Schaffer M. Curcumin as an anticancer agent: review of the gap between basic and clinical applications. Curr Med Chem 2010;17:190–197.
Henrotin Y, Clutterbuck AL, Allaway D. Biological actions of curcumin on articular chondrocytes. Osteoarthrtis Cartilage 2010;18:141–149.
Li WQ, Dehnade F, Zafarullah M. Oncostatin M-induced matrix metalloproteinase and tissue inhibitor of metalloproteinase-3 genes expression in chondrocytes requires Janus kinase/STAT signaling pathways. J Immunol 2001;166:3491–3498.
Schulze-Tanzil G, Mobasheri A, Sendzik J, John T, Shakibaei M. Effects of curcumin (diferuloylmethane) on nuclear factor κB signaling in interleukin-1β-stimulated chondrocytes. Ann N Y Acad Sci 2004;1030:578–586.
Shakibaei M, Schulze-Tanzil G, John T, Mobasheri A. Curcumin protects human chondrocytes from IL-1β-induced inhibition of collagen type II and β1-integrin expression and activation of caspase-3: an immunomorphological study. Ann Anat 2005;187:487–497.
Sylvester J, Liacini A, Li WQ, Zafarullah M. Interleukin-17 signal transduction pathways implicated in inducing matrix metalloproteinase-3, -13 and aggrecanase-1 genes in articular chondrocytes. Cell Signal 2004;16:469–476.
Cho ML, Jung YO, Moon YM, Min SY, Yoon CH, Lee SH, et al. Interleukin-18 induces the production of vascular endothelial growth factor (VEGF) in rheumatoid arthritis synovial fibroblasts via AP-1-dependent pathways. Immunol Lett 2006;103:159–166.
Hong J, Bose M, Ju J, Ryu JH, Chen X, Sang S, et al. Modulation of arachidonic acid metabolism by curcumin and related β-diketone derivatives: effects on cytosolic phospholipase A2, cyclooxygenases and 5-lipoxygenase. Carcinogenesis 2004;25:1671–1679.
Brouet I, Ohshima H. Curcumin, an anti-tumour promoter and anti-inflammatory agent, inhibits induction of nitric oxide synthase in activated macrophages. Biochem Biophys Res Commun 1995;206:533–540.
Cho JW, Lee KS, Kim CW. Curcumin attenuates the expression of IL-1β, IL-6, and TNF-α as well as cyclin E in TNF-α-treated HaCaT cells; NF-κB and MAPKs as potential upstream targets. Int J Mol Med 2007;19:469–474.
Elmali N, Baysal O, Harma A, Esenkaya I, Mizrak B. Effects of resveratrol in inflammatory arthritis. Inflammation 2007;30:1–6.
Hou Y, Wu J, Huang Q, Guo L. Luteolin inhibits proliferation and affects the function of stimulated rat synovial fibroblasts. Cell Biol Int 2009;33:135–147.
Moncada-Pazos A, Obaya AJ, Viloria CG, López-Otín C, Cal S. The nutraceutical flavonoid luteolin inhibits ADAMTS-4 and ADAMTS-5 aggrecanase activities. J Mol Med 2011;89:611–619.
Wang J, Zhang Q, Jin S, He D, Zhao S, Liu S. Genistein modulate immune responses in collagen-induced rheumatoid arthritis model. Maturitas 2008;59:405–412.
Sato M, Miyazaki T, Kambe F, Maeda K, Seo H. Quercetin, a bioflavonoid, inhibits the induction of interleukin 8 and monocyte chemoattractant protein-1 expression by tumor necrosis factor-alpha in cultured human synovial cells. J Rheumatol 1997;24:1680–1684.
Fernández López JC, Ruano-Ravina A. Efficacy and safety of intraarticular hyaluronic acid in the treatment of hip osteoarthritis: a systematic review. Osteoarthritis Cartilage 2006;14:1306–1311.
Reichenbach S, Blank S, Rutjes AW, Shang A, King EA, Dieppe PA, et al. Hylan versus hyaluronic acid for osteoarthritis of the knee: a systematic review and metaanalysis. Arthritis Rheum 2007;57:1410–1418.
Julovi SM, Yasuda T, Shimizu M, Hiramitsu T, Nakamura T. Inhibition of interleukin-1β-stimulated production of matrix metalloproteinases by hyaluronan via CD44 in human articular cartilage. Arthritis Rheum 2004;50:516–525.
Wang CT, Lin YT, Chiang BL, Lin YH, Hou SM. High molecular weight hyaluronic acid down-regulates the gene expression of osteoarthritis-associated cytokines and enzymes in fibroblast-like synoviocytes from patients with early osteoarthritis. Osteoarthritis Cartilage 2006;14:1237–1247.
Takahashi K, Goomer RS, Harwood F, Kubo T, Hirasawa Y, Amiel D. The effects of hyaluronan on matrix metalloproteinase-3 (MMP-3), interleukin-1β (IL-1β), and tissue inhibitor of metalloproteinase-1 (TIMP-1) gene expression during the development of osteoarthritis. Osteoarthritis Cartilage 1999;7:182–190.
Yatabe T, Mochizuki S, Takizawa M, Chijiiwa M, Okada A, Kimura T, et al. Hyaluronan inhibits expression of ADAMTS4 (aggrecanase-1) in human osteoarthritic chondrocytes. Ann Rheum Dis 2009;68:1051–1058.
Dougados M. Symptomatic slow-acting drugs for osteoarthritis: what are the facts? Joint Bone Spine 2006;73:606–609.
Lamari FN. The potential of chondroitin sulfate as a therapeutic agent. Connect Tissue Res 2008;49:289–292.
Imada K, Oka H, Kawasaki D, Miura N, Sato T, Ito A. Antiarthritic action mechanisms of natural chondroitin sulfate in human articular chondrocytes and synovial fibroblasts. Biol Pharm Bull 2010;33:410–414.
Legendre F, Baugé C, Roche R. Chondroitin sulfate modulation of matrix and inflammatory gene expression in IL-1β-stimulated chondrocytes-study in hypoxic alginate bead cultures. Osteoarthritis Cartilage 2008;16:105–114.
Tahiri K, Korwin-Zmijowska C, Richette P, Héraud F, Chevalier X, Savouret JF, et al. Natural chondroitin sulphates increase aggregation of proteoglycan complexes and decrease ADAMTS-5 expression in interleukin-1β-treated chondrocytes. Ann Rheum Dis 2008;67:696–702.
Kumagai K, Shirabe S, Miyata N, Murata M, Yamauchi A, Kataoka Y, et al. Sodium pentosan polysulfate resulted in cartilage improvement in knee osteoarthritis-an open clinical trial. BMC Clin Pharmacol 2010;10:7.
Ghosh P. The pathobiology of osteoarthritis and the rationale for the use of pentosan polysulfate for its treatment. Semin Arthritis Rheum 1999;28:211–267.
Troeberg L, Fushimi K, Khokha R, Emonard H, Ghosh P, Nagase H. Calcium pentosan polysulfate is a multifaceted exosite inhibitor of aggrecanases. FASEB J 2008;22:3515–3524.
Takizawa M, Yatabe T, Okada A, Chijiiwa M, Mochizuki S, Ghosh P, et al. Calcium pentosan polysulfate directly inhibits enzymatic activity of ADAMTS4 (aggrecanase-1) in osteoarthritic chondrocytes. FEBS Lett 2008;582:2945–2949.
Yang YH, Rajaiah R, Lee DY, Ma Z, Yu H, Fong HH, et al. Suppression of ongoing experimental arthritis by a Chinese herbal formula (Huo-Luo-Xiao-Ling Dan) involves changes in antigen-induced immunological and biochemical mediators of inflammation. Evid Based Complement Altern Med 2011:642027.
Li F, Wu H, Deng JW, Fan SQ, Tian J, Gao JS, et al. Effect of Yangqixue Qufengshi Recipe on rheumatoid arthritis model mice under different genetic backgrounds. Chin J Integr Med 2006;12:46–49.
Wang LR, Ishiguro N, Yamada E, Nishida Y, Sato K, Iwata H. The effect of Da-Fang-Feng-Tang on treatment of type II collagen-induced arthritis in DBA/1 mice. Am J Chin Med 1 1999;27:205–215.
Yang M, Xiao C, Wu Q, Niu M, Yao Q, Li K, et al. Antiinflammatory effect of Sanshuibaihu Decoction may be associated with nuclear factor-κB and p38 MAPKα in collagen-induced arthritis in rat. J Ethonopharmacol 2010;127:264–273.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Keisuke, I., Bian, Bl., Li, Xd. et al. Action mechanisms of complementary and alternative medicine therapies for rheumatoid arthritis. Chin. J. Integr. Med. 17, 723–730 (2011). https://doi.org/10.1007/s11655-011-0871-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11655-011-0871-3