Abstract
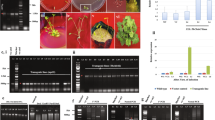
Controlling the expression of genes related to plant defense mechanisms is crucial when engineering plants with increased resistance to pathogens. In this study, synthetic promoters were placed upstream of a chimeric chitinase defense gene to produce transformation vectors. Canola plants were transformed with three constructs, pGDEC, pGMPC, pBISM2, containing synthetic promoters, synthetic pathogen-inducible promoter (SP-DDEE; parsley D and E17 elements + minimal promoter), SP-MP (minimal promoter), and the CaMV35S constitutive promoter, respectively. The results of reverse transcriptase PCR (RT-PCR) and enzyme activity assays show that the synthetic pathogen-inducible promoter (SP-DDEE) was responsive to the chitin and fungal elicitors, whereas negative control pGMPC did not respond. Furthermore promoters were assessed in the transgenic lines for antifungal activity against two phytopathogenic fungi. Results indicated that total proteins from transgenic lines carrying the SP-DDEE promoter when treated with elicitors strongly inhibited fungal growth, particularly Sclerotinia sclerotiorum, the major pathogen of canola. Overall, results indicate that not only was the SP-DDEE synthetic promoter highly responsive to the pathogen elicitors, but the inducible expression of the chimeric chitinase gene, when controlled by the SP-DDEE promoter, was also adequate to inhibit the fungal growth and development.








Similar content being viewed by others
References
Broekaert WF, Delaure SL, De Bolle MFC, Cammue BPA (2006) The role of ethylene in host-pathogen interactions. Annu Rev Phytopathol 44:393–416
Brogue K, Chet I, Holliday M, Cressman R, Biddle P, Knowlton S, Mauvais CJ, Broglie R (1991) Transgenic plants with enhanced resistance to the fungal pathogen Rhizoctonia solani. Science 254(5035):1194–1197
Butaye KM, Cammue BP, Delauré SL, De Bolle MF (2005) Approaches to minimize variation of transgene expression in plants. Mol Breed 16:79–91
Cazzonelli CI, Velten J (2008) In vivo characterization of plant promoter element interaction using synthetic promoters. Transgenic Res 17(3):437–457
Chan Y-L, He Y, Hsiao T-T, Wang C-J, Tian Z, Yeh K-W (2015) Pyramiding taro cystatin and fungal chitinase genes driven by a synthetic promoter enhances resistance in tomato to root-knot nematode Meloidogyne incognita. Plant Sci 231:74–81
Dey N, Sarkar S, Acharya S, Maiti IB (2015) Synthetic promoters in planta. Planta 242:1077–1094
Eulgem T, Rushton PJ, Robatzek S, Somssich IE (2000) The WRKY superfamily of plant transcription factors. Trends Plant Sci 5:199–206
Gunaratna KR, Balasubramanian R (1994) Partial purification and properties of extracellular chitinase produced by Acremonium obclavatum, an antagonist to the groundnut rust, Puccinia arachidis. World J Microbiol Biotechnol 10(3):342–345
Gurr SJ, Rushton PJ (2005) Engineering plants with increased disease resistance: what are we going to express? Trends Biotechnol 23(6):275–282
Hammond-Kosack KE, Parker JE (2003) Deciphering plant pathogen communication: fresh perspectives for molecular resistance breeding. Curr Opin Biotechnol 14(2):177–193
Heise A, Lippok B, Kirsch C, Hahlbrock K (2002) Two immediate-early pathogen-responsive members of the AtCMPG gene family in Arabidopsis thaliana and the W-box-containing elicitor-response element of AtCMPG1. Proc Natl Acad Sci 99:9049–9054
Hu CH, Wei YR, Huang YH, Yi GJ (2013) An efficient protocol for the production of chit42 transgenic Furenzhi banana (Musa spp. AA group) resistant to Fusarium oxysporum. In Vitro Cell Dev Biol-Plant 49(5):584–592
Joosten M, Verbakel H, Nettekoven M, Van Leeuwen J, Van der Vossen R, De Wit P (1995) The phytopathogenic fungus Cladosporium fulvum is not sensitive to the chitinase and β-1, 3-glucanase defence proteins of its host, tomato. Physiol Mol Plant Pathol 46(1):45–59
Kirsch C, Logemann E, Lippok B, Schmelzer E, Hahlbrock K (2001) A highly specific pathogen-responsive promoter element from the immediate-early activated CMPG1 gene in Petroselinum crispum. Plant J 26:217–227
Kojima M, Yoshikawa T, Ueda M, Nonomura T, Matsuda Y, Toyoda H, Miyatake K, Arai M, Fukamizo T (2005) Family 19 chitinase from Aeromonas sp. No. 10S-24: role of chitin-binding domain in the enzymatic activity. J Biochem 137(2):235–242
Kunz C, Sellam O, Bertheau Y (1992) Purification and characterization of a chitinase from the hyperparasitic fungus Aphanocladium album. Physiol Mol Plant Pathol 40(2):117–131
Lebel E, Heifetz P, Thorne L, Uknes S, Ryals J, Ward E (1998) Functional analysis of regulatory sequences controlling PR-1 gene expression in Arabidopsis. Plant J 16:223–233
Limon MC, Chacon MR, Mejias R, Delgado-Jarana J, Rincon AM, Codon AC, Benitez T (2004) Increased antifungal and chitinase specific activities of Trichoderma harzianum CECT 2413 by addition of a cellulose binding domain. Appl Microbiol Biotechnol 64(5):675–685
Liu H, Guo X, Naeem MS, Liu D, Xu L, Zhang W, Tang G, Zhou W (2010) Transgenic Brassica napus L. lines carrying a two gene construct demonstrate enhanced resistance against Plutella xylostella and Sclerotinia sclerotiorum. Plant Cell Tissue Organ Cult 106:143–151
Liu H, Naeem MS, Liu D, Zhu Y, Guo X, Cui P, Zhou W (2011) Analyses of inheritance patterns and consistent expression of sporamin and chitinase PjChi-1 genes in Brassica napus. Plant Breed 130:345–351
Lorito M, Woo SL, Fernandez IG, Colucci G, Harman GE, Pintor-Toro JA, Filippone E, Muccifora S, Lawrence CB, Zoina A, Tuzun S, Scala F (1998) Genes from mycoparasitic fungi as a source for improving plant resistance to fungal pathogens. Proc Natl Acad Sci 95(14):7860–7865
Matroodi S, Motallebi M, Zamani M, Moradyar M (2013) Designing a new chitinase with more chitin binding and antifungal activity. World J Microbiol Biotechnol 29(8):1517–1523
Mauch F, Mauch-Mani B, Boller T (1988) Antifungal hydrolases in pea tissue II. Inhibition of fungal growth by combinations of chitinase and β-1, 3-glucanase. Plant Physiol 88(3):936–942
Mazarei M, Teplova I, Hajimorad MR, Stewart CN (2008) Pathogen phytosensing: plants to report plant pathogens. Sensors 8(4):2628–2641
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15(3):473–497
Niemeyer J, Ruhe J, Machens F, Stahl DJ, Hehl R (2014) Inducible expression of p50 from TMV for increased resistance to bacterial crown gall disease in tobacco. Plant Mol Biol 84:111–123
Nurnberger T, Brunner F (2002) Innate immunity in plants and animals: emerging parallels between the recognition of general elicitors and pathogen-associated molecular patterns. Curr Opin Plant Biol 5:318–324
Potenza C, Aleman L, Sengupta-Gopalan C (2004) Targeting transgene expression in research, agricultural, and environmental applications: promoters used in plant transformation. Vitro Cell Dev Biol-Plant 40(1):1–22
Qing CM, Fan L, Lei Y, Bouchez D, Tourneur C, Yan L, Robaglia C (2000) Transformation of Pakchoi (Brassica rapa L. ssp. chinensis) by Agrobacterium infiltration. Mol Breed 6(1):67–72
Rogers SO, Bendich AJ (1985) Extraction of DNA from milligram amounts of fresh, herbarium and mummified plant tissues. Plant Mol Biol 5(2):69–76
Rushton PJ, Reinstadler A, Lipka V, Lippok B, Somssich IE (2002) Synthetic plant promoters containing defined regulatory elements provide novel insights into pathogen-and wound-induced signaling. Plant Cell 14(4):749–762
Rushton PJ, Somssich IE (1998) Transcriptional control of plant genes responsive to pathogens. Curr Opin Plant Biol 1:311–315
Salinas J, Oeda K, Chua NH (1992) Two G-box-related sequences confer different expression patterns in transgenic tobacco. Plant Cell 4(12):1485–1493
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor, New York
Shokouhifar F, Zamani M, Motallebi M, Mousavi A, Malboobi M (2011) Construction and functional analysis of pathogen-inducible synthetic promoters in Brassica napus. Biol Plant 55:689–695
Venter M (2007) Synthetic promoters: genetic control through cis engineering. Trends Plant Sci 12(3):118–124
Zeilinger S, Galhaup C, Payer K, Woo SL, Mach RL, Fekete C, Loritob M, Kubiceka CP (1999) Chitinase gene expression during mycoparasitic interaction of Trichoderma harzianum with its host. Fugal Gent Biol 26(2):131–140
Zou X, Song E, Peng A, He Y, Xu L, Lei T, Yao L, Chen S (2014) Activation of three pathogen-inducible promoters in transgenic citrus (Citrus sinensis Osbeck) after Xanthomonas axonopodis pv. citri infection and wounding. Plant Cell Tissue Organ Cult 117:85–98
Acknowledgment
The authors would like to thank Akram Zamani for contributing in editing of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: Ewen Mullins
Rights and permissions
About this article
Cite this article
Moradyar, M., Motallebi, M., Zamani, M.R. et al. Pathogen-induced expression of chimeric chitinase gene containing synthetic promoter confers antifungal resistance in transgenic canola. In Vitro Cell.Dev.Biol.-Plant 52, 119–129 (2016). https://doi.org/10.1007/s11627-016-9751-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-016-9751-z