Abstract
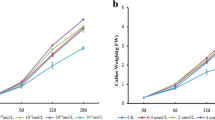
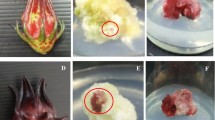
Gynura bicolor DC., a traditional vegetable in Japan, is cultivated as Kinjisou and Suizenjina in Ishikawa and Kumamoto prefectures, respectively. The adaxial side of the leaves of G. bicolor grown in a field is green, and the abaxial side is reddish purple. It has been reported that these reddish purple pigments are anthocyanins. Although we established a culture system of G. bicolor, the leaves of G. bicolor plants grown under our culture conditions showed green color on both sides of all leaves. We investigated the effects of phytohormones and chemical treatments on anthocyanin accumulation in cultured plants. Although anthocyanin accumulation in the leaves was slightly stimulated, anthocyanins accumulation in the roots of the cultured plant was induced remarkably by 25–50 μM methyl jasmonate (MJ) treatment. This induction was affected by light irradiation and sucrose concentration in the culture medium. However, salicylic acid (SA) and 1-aminocyclopropane-1-carboxylic acid did not induce anthocyanin accumulation in roots. And then, combinations of MJ and SA or MJ and AgNO3 did not stimulate the anthocyanin accumulation in the root as found in the case of treatment by MJ solely.







Similar content being viewed by others
References
Adam M. T.; Felix W. J.; Steele R. J.; Jochen B.; Simon R. P.; Amanda R. W. Light-induced expression of a MYB gene regulates anthocyanin biosynthesis in red apples. Plant Physiol 142: 1216–1232; 2006.
Balbi V.; Devoto A. Jasmonate signalling network in Arabidopsis thaliana: crucial regulatory nodes and new physiological scenarios. New Phytol 177: 301–318; 2008.
Belhadj A.; Telef N.; Saigne C.; Cluzet S.; Barrieu F.; Hamdi S.; Mérillon J. M. Effect of methyl jasmonate in combination with carbohydrates on gene expression of PR proteins, stilbene and anthocyanin accumulation in grapevine cell cultures. Plant Physiol Biochem 46: 493–499; 2008.
Chalker-Scott L. Environmental significance of anthocyanins in plant stress responses. Photochem Photobiol 70: 1–9; 1999.
Curtin C.; Zhang W.; Franco C. Manipulationg anthocyanin composition in Vitis vinifera suspension cultures by elicitation with jasmonic acid and light irradiation. Biotechnol Let 25: 1131–1135; 2003.
Dong Y.; Beuning L.; Davies K.; Mitra D.; Morris B.; Kootstra A. Expression of pigmentation genes and photo-regulation of anthocyanin biosynthesis in developing Royal Gala apple flowers. J Plant Physiol 25: 245–252; 1998.
Dixon R. A.; Paiva N. L. Stress-induced phyenylpropanoid metabolism. Plant Cell 7: 1085–1097; 1995.
Feinbaum R.; Ausubel F. Transcriptional regulation of the Arabidopsis thaliana chalcone synthase gene. Mol Cell Biol 8: 1985–1992; 1998.
Gong Z.; Yamazaki M.; Saito K. A. Light-inducible Myb-like gene that is spscifically expressed in red Perilla frutescens and presumably acts as a determining factor of the anthocyanin forma. Mol Gen Genet 262: 65–72; 1999.
Gould K. S.; Neill S. O.; Vogelmann T. C. A unified explanation for anthocyanins in leaves. AdvBo Res 37: 167–192; 2002.
Harborne J. B.; Williams C. A. Advances in flavonoid research since 1992. Phytochemistry 55: 481–504; 2000.
Hayashi M.; Ota R.; Miwa S.; Takenaka S.; Enomoto T. Food chemical properties of Kinjisou coloring matter and the structure of anthocyanin. Bull Ishikawa Agric Res Cntr Jpn 26: 31–35; 2005.
KakegawaK K. Y.; Hattori E.; Koike K.; Taeda K. Cell cultures of Centaurea cyanus produce malonate anthocyanin in UV light. Phytocemistry 26: 2261–2263; 1987.
Loreti E.; Povero G.; Novi G.; Solfanelli C.; Alpi A.; Perata P. Gibberellins, jasmonate and abscisic acid modulate the sucrose-induced expression of anthocyanin biosynthetic genes in Arabidopsis. New Phytol 179: 1004–1016; 2008.
Murashige T.; Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15: 473–497; 1962.
Nagira Y.; Ikegami K.; Koshiba T.; Ozeki Y. Effect of ABA upon anthocyanin synthesis in regenerated torenia shoots. J Plant Res 119: 137–144; 2006.
Nagira Y.; Ozeki Y. A system in which anthocyanin synthesis is induced in regenerated torenia shoots. J Plant Res 117: 377–383; 2004.
Plata N.; Konczak-Islam I.; Jayram S.; McCelland K.; Woolford T.; Franks P. Effect of methyl jasmonate and p-coumaric acid on anthocyanin composition in a sweet potato cell suspension culture. Biochmeical Eng J 14: 171–177; 2003.
Pozo M. J.; Van Loon L. C.; Pieterse C. M. Jasmonates signals in plant-microbe interactions. J Plant Growth Regul 23: 211–222; 2005.
Rudell D. R.; Mattheis J. P. Synergism exists between ethylene and methyl jasmonate in artificial light-induced pigment enhancement of ‘Fuji’ apple fruit peel. Postharvest Biol Technol 47: 136–140; 2008.
Saniewski M.; Miyamoto K.; Ueda J. Methyl jasmonate induces gums and stimulates anthocyanin accumulation in peach shoots. J Plant Growth Regul 17: 121–124; 1998.
Seo S.; Sano H.; Ohashi Y. Jasmonaic acid in wound signal transduction pathways. Physiol Plant 101: 740–745; 1997.
Yoshitama K.; Kaneshige M.; Ishikura N.; Araki F.; Yahara S.; Abe K. A stable reddish purple anthocyanin in the leaf of Gynura aurantiaca cv. ‘Purple Passion’. J Plant Res 107: 209–214; 1994.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: S. J. Murch
Rights and permissions
About this article
Cite this article
Shimizu, Y., Maeda, K., Kato, M. et al. Methyl jasmonate induces anthocyanin accumulation in Gynura bicolor cultured roots. In Vitro Cell.Dev.Biol.-Plant 46, 460–465 (2010). https://doi.org/10.1007/s11627-010-9294-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-010-9294-7