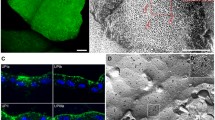
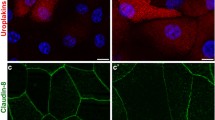
Abstract
Tight junctions (TJs) are essential for normal function of epithelia, restricting paracellular diffusion and contributing to the maintenance of cell surface polarity. Superficial cells of the urothelium develop TJs, the basis for the paracellular permeability barrier of the bladder against diffusion of urinary solutes. Focusing on the superficial cell layer of stratified cell cultures of an immortalized human ureteral cell line, TEU-2 cells, we have examined the presence of TJ and TJ-associated proteins. TEU-2 cells were treated with calcium chloride and fetal bovine serum culture conditions used to induce stratification that resembles the normal transitional epithelial phenotype. Cultures were examined for TJ and TJ-associated proteins by confocal immunofluorescence microscopy and evaluated for TJ mRNA by reverse transcriptase-polymerase chain reaction (RT-PCR). TEU-2 cultures exhibited immunoreactivity at intercellular margins for claudins 1, 4, 5, 7, 14, and 16 whereas claudins 2, 8, and 12 were intracellular. RT-PCR corroborated the presence of these claudins at the mRNA level. The TJ-associated proteins occludin, JAM-1, and zonula occludens (ZO-1, ZO-2, and ZO-3) were localized at cell margins. We have found that numerous TJs and TJ-associated proteins are expressed in stratified TEU-2 cultures. Further, we propose TEU-2s provide a useful ureteral model for future studies on the involvement of TJs proteins in the normal and pathological physiology of the human urinary system.



Similar content being viewed by others
References
Acharya P.; Beckel J.; Ruiz W. G.; Wang E.; Rojas R.; Birder L.; Apodaca G. Distribution of the tight junction proteins ZO-1, occludin, and claudin-4, -8, and -12 in bladder epithelium. Am. J. Physiol. Renal. Physiol. 287: F305–F318; 2004.
Aijaz S.; Balda M. S.; Matter K. Tight junctions: molecular architecture and function. Int. Rev. Cytol. 248: 261–298; 2006.
Angelow S.; Kim K. J.; Yu A. S. L. Claudin-8 modulates paracellular permeability to acidic and basic ions in MDCK II cells. J. Physiol. 571: 15–26; 2006.
Cross W. R.; Eardley I.; Leese H. J.; Southgate J. A biomimetic tissue from cultured normal human urothelial cells: Analysis of physiological function. Am. J. Physiol. Renal. Physiol. 289: F459–F468; 2005.
Furuse M.; Furuse K.; Sasaki H.; Tsukita S. Conversion of zonulae occludentes from tight to leaky strand type by introducing claudin-2 into Madin-Darby canine kidney I cells. J. Cell. Biol. 153: 263–272; 2001.
Furuse M.; Hirase T.; Itoh M.; Nagafuchi A.; Yonemura S.; Tsukita S.; Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J. Cell. Biol. 123: 1777–1788; 1993.
Klumpp D. J.; Wieser A. C.; Sengupta S.; Forrestal S. G.; Batler R. A.; Schaeffer A. J. Uropathogenic E. coli potentiates type I pilus-induced apopotosis by suppressing NF-κB. Infect. Immun. 69: 6689–6695; 2001.
Lewis S. A. Everything you wanted to know about the bladder epithelium but were afraid to ask. Am. J. Physiol. Renal. Physiol. 278: F867–F874; 2000.
Li W. Y.; Huey C. L.; Yu A. S. L. Expression of claudin-7 and -8 along the mouse nephron. Am. J. Physiol. Renal. Physiol. 286: F1063–F1071; 2004.
McCarthy K. M.; Francis S. A.; McCormack J. M.; Lai J.; Rogers R. A.; Skare I. B.; Lynch R. D.; Schneeberger E. E. Inducible expression of claudin-1-myc but not occludin-VSV-G results in aberrant tight junction strand formation in MDCK cells. J. Cell. Sci. 113: 3387–3398; 2000.
McCarthy K. M.; Skare I. B.; Stankewich M. C.; Furuse M.; Tsukita S.; Rogers R. A.; Lynch R. D.; Schneeberger E. E. Occludin is a functional component of the tight junction. J. Cell. Sci. 109: 2287–2298; 1996.
Rangel L. B. A.; Sherman-Baust C. A.; Wernyj R. P.; Schwartz D. R.; Cho K. R.; Morin P. J. Characterization of novel human ovarian cancer-specific transcripts (HOSTs) identified by serial analysis of gene expression. Oncogene 22: 7225–7232; 2003.
Saitou M.; Furuse M.; Sasaki H.; Schulzke J. D.; Fromm M.; Takano H.; Noda T.; Tsukita S. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol. Biol. Cell. 11: 4131–4142; 2000.
Simon D. B.; Lu Y.; Choate K. A.; Velazquez H.; Al-Sabban E.; Praga M.; Casari G.; Bettinelli A.; Colussi G.; Rodriquez-Soriano J.; McCredie D.; Milford D.; Sanjad S.; Lifton R. P. Paracellin-1, a renal tight junction protein required for paracellular Mg (2+) resorption. Science 285: 103–106; 1999.
Southgate J.; Hutton K. A. R.; Thomas D. F. M.; Trejdosiewicz L. K. Normal human urothelial cells in vitro: proliferation and induction of stratification. Lab. Invest. 71: 583–594; 1994.
Turksen K.; Troy T. C. Barriers built on claudins. J. Cell. Sci. 117: 2435–2447; 2004.
Van Itallie C.; Rahner C.; Anderson J. M. Regulated expression of claudin-4 decreases paracellular conductance through a selective decrease in sodium permeability. J. Clin. Invest. 107: 1319–1327; 2001.
Varley C. L.; Garthwaite M. A. E.; Cross W.; Hinley J.; Trejdosiewicz L. K.; Southgate J. PPARγ-regulated tight junction development during human urothelial cytodifferentiation. J. Cell. Physiol. 208: 407–417; 2006.
Yu A. S. L.; McCarthy K. M.; Francis S. A.; McCormack J. M.; Lai J.; Rogers R. A.; Lynch R. D.; Schneeberger E. E. Knockdown of occludin expression leads to diverse phenotypic alterations in epithelial cells. Am. J. Physiol. Cell. Physiol. 288: C1231–C1241; 2005.
Wilcox E. R.; Burton Q. L.; Naz S.; Riazuddin S.; Smith T. N.; Ploplis B.; Belyantseva I.; Ben-Yosef T.; Liburd N. A.; Morell R. J.; Kachar B.; Wu D. K.; Griffith A. J.; Riazuddin S.; Friedman T. B. Mutations in the gene encoding tight junction claudin-14 cause autosomal recessive deafness DFNB29. Cell 104: 165–172; 2001.
Acknowledgement
Supported by National Institute of Diabetes and Digestive and Kidney Diseases Award DK66119.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: J. Denry Sato
Rights and permissions
About this article
Cite this article
Rickard, A., Dorokhov, N., Ryerse, J. et al. Characterization of tight junction proteins in cultured human urothelial cells. In Vitro Cell.Dev.Biol.-Animal 44, 261–267 (2008). https://doi.org/10.1007/s11626-008-9116-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-008-9116-y