Abstract
Aim
This study investigated whether an individual’s age at diagnosis of hypertension, which is associated with a decline in cognitive performance in the China Health and Retirement Longitudinal Study (CHARLS) participants.
Methods
Our analysis was based on the CHARLS with baseline data collected between 2011 and 2018. We randomly selected a control participant for each hypertensive participant using propensity score. The cohort comprised 2413 individuals with hypertension and 2411 controls. Participants were divided into three groups as follows: non-hypertension, hypertension diagnose ≥55 years, and hypertension diagnose <55 years. Cognitive performance was measured in both visits and evaluated by the scores of the memory, executive function, and orientation and global cognitive.
Results
After multivariable adjustment, individuals with hypertension diagnosed <55 years had a significantly faster cognitive decline in memory test (β (95% CI, −1.117 [−1.405, −0.83]), orientation test (β (95% CI, −1.273 [−1.348, −1.198]) and global cognitive (β (95% CI, −1.611 [−1.744, −1.478]) compared with the corresponding controls. A longer hypertension duration was associated with worse memory test (β (95% CI, −0.069 [−0.113 to −0.025]). Among treated individuals, blood pressure control at baseline was inversely associated with the decline in orientation test (β (95% CI, −0.659 [−0.939, −0.380]), orientation test (β (95% CI, −0.259[−0.365, −0.153])and global cognitive (β (95% CI, −0.124 [−0.162, −0.086]).
Conclusions
Our findings suggest that hypertension diagnosed in mid-life is associated with worse cognition compared to late life. Besides, longer duration of diagnosis is associated with worse memory test. In addition to hypertension, pressure control might be critical for the preservation of cognitive function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is estimated that the number of adults with hypertension worldwide was 1.4 billion in 2010 and may increase to 1.6 billion by 2025.1 The latest epidemiological investigation in China showed that the hypertension prevalence in the old population was more than 20%.2 Accumulating epidemiologic and mechanistic evidence suggests that hypertension is implicated in the occurrence of cognitive decline,3,4 and is potentially preventable and treatable.5,6,7 Although it is not known exactly when hypertension begins to affect cognition, findings from previous studies have shown middle age seems to be a sensitive period in which exposure to hypertension has a subtle negative impact on the brain more than hypertension started at older ages.8,9,10,11 In addition, the use of antihypertensive medications12,13 and control of stress levels14 appear to moderate the association between high blood pressure and cognitive decline. Among Chinese adults, nearly half have hypertension, fewer than a third are being treated, and fewer than one in twelve are in control of their blood pressure.2 National data on the association of age at diagnosis of hypertension with cognitive in China are scarce, and how hypertension awareness, treatment, and control rates vary geographically and across population subgroups affect the association is uncertain.
Studies investigating the association between hypertension duration and cognitive have yielded mixed results. Some studies have suggested an association between longer duration of hypertension and an increased risk of cognitive decline and dementia,15,16 whereas others showed no association.17 On the other hand, earlier onset of hypertension means longer duration of exposure to hypertension, regardless of age at onset of hypertension. Therefore, the shorter duration of hypertension may partly explain the inconsistency between studies examining the effects of hypertension on cognitive decline or dementia in older adults.16 Given these conflicting results, age of diagnosis and duration of hypertension must be addressed.
A better understanding of the effect of age on the association of hypertension and the duration of hypertension is important for promoting cognitive health. Herein, using a population-based cohort, we sought to evaluate the association between age at diagnosis of hypertension at different stages of life-course, hypertension duration, control of hypertension, and cognitive function after an 8-year follow-up.
Methods
Study Population
This study used data from waves 1 to 4 (2011 to 2018) of the China Health Retirement Longitudinal Study (CHARLS), a multicenter cohort of randomly selected Chinese residents aged 45 years or older from both urban and rural areas in 28 provinces. The data are available online at https://charls.pku.edu.cn/. The cohort was conformed to the Declaration of Helsinki and approved by the Peking University Institutional Review Board (IRB00001052-11015). Detailed information on the study was previously published.18,19 All participants signed an informed consent.
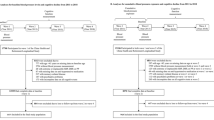
As shown in Fig. 1, among the 17,708 participants interviewed in the baseline survey, a total of 11,868 individuals completed the three subsequent follow-ups. Wave 1 (2011) in the CHARLS was regarded as the baseline in the present study. One control for each individual with hypertension was randomly selected from those without hypertension at baseline using propensity score. Our propensity score accounted for age, sex, education, alcohol consumption, physical activity, smoking, body mass index, and depression (Fig. 1). Consistent with the previous studies,20,21 individuals with average systolic blood pressure (SBP) < 140 mmHg and average diastolic blood pressure (DBP) < 90 mmHg and who did not use antihypertensive were classified as normal and hypertension was defined as (1) SBP ≥ 140 mmHg or average DBP ≥ 90mmHg, or (2) self-reported use of antihypertension agents (either chemical drugs or traditional Chinese herbal products) at the time of investigation. In addition, we excluded individuals because of (1) missing diagnosis age at hypertension (n=1532) and (2) missing date of birth (n=4).
Age at Diagnosis of Hypertension
Participants were asked whether they had ever been told by a doctor that they had hypertension. For those who reported a diagnosis of hypertension, they were requested to answer a further question “What was your age when hypertension was first diagnosed?” The following checks were performed: (1) reject if answer <0; (2) reject if answer >participant’s age; (3) ask to confirm if the answer. The age of diagnosis was then categorized as (1) middle-age hypertension (<55 years) and (2) late hypertension (≥55 years). Duration of hypertension (visit 1 age-age of hypertension diagnosis) and analyzed as a continuous variable.
Blood Pressure
BP was measured using a validated oscillometric device (Omron HEM 705CPINT) on the right arm after a 5-min rest in a sitting position in a quiet, temperature-controlled room (20–24 °C). Three measurements were taken at 1-min intervals, and the means of the three measurements of SBP and DBP were used.
The effect of antihypertensive use per se on cognitive performance was evaluated on participants with hypertension diagnosis before study entry (N=2413) and grouped into control and uncontrol. The role of BP control was evaluated only in hypertensive individuals under treatment at visit 1 (N=1813) and categorized into controlled hypertension (SBP<140 mm Hg and DBP<90 mm Hg) and uncontrolled hypertension (SBP≥140 mm Hg or DBP≥90 mm Hg).
Cognitive Evaluation
Cognitive assessments were conducted in waves 1 to 4 in the CHARLS. Three cognitive domains were covered, including memory, executive function, and orientation. The first one is episodic memory through immediate and delayed recall. Ten unrelated Chinese words were read to each participant, and the memory ability was evaluated by counting how many words could be recalled immediately (immediate word recall) and 4 min later (delayed word recall). One point was given for each word recalled in either an immediate or a delayed recall task (0 to 20 points). Executive function was assessed by an intersecting pentagon copying test (0 or 3 points) and the Serial Sevens test (0 to 5 points) in the CHARLS (0 to 8 points in total). In the intersecting pentagon copying test, participants were asked to observe and then draw a picture of two overlapping pentagons. A successful drawing was given three points. In the Serial Sevens test, participants counted backwards from 100 by sevens for five times. One point was given for each correct answer. Orientation was evaluated by four questions on the year, the month, the date of the month, and the day of the week. The sum of correct answers was regarded as the orientation score (0 to 4 points). Both the validity and the reliability of these tests have been well documented.22 To assess overall cognitive function, z-scores were generated for each cohort in two steps: step 1—normalize to baseline, generate domain z-score. Each range test score was subtracted from the mean and then divided by the standard deviation (SD) of the baseline scores. For example, in CHARLS, the mean and standard deviation of baseline executive function were 5.4 and 2.4, respectively. Then calculate the performer Z-score for each wave (original performer score—5.4)/2.4. Step 2—renormalize the mean of the three domains to the baseline and calculate the individual global z-scores in each wave. This method of generating cognitive z-scores is well established.23,24,25
Covariables
Sociodemographic characteristics included age, sex, and education. The health behaviors included cohabitation status, smoking (nonsmoker, ex-smoker, and smoker), leisure physical activity, sleep duration, and alcohol consumption. Cohabitation status was categorized as living alone or not. Once per week or a higher frequency of drinking was defined as alcohol consumption. Engaging in vigorous or moderate activities once weekly or more was regarded as physically active. Depressive symptoms were evaluated by an 8-item version of the Center for Epidemiologic Studies Depression a 10-item version of the CESD (CESD-10, 0 to 3 points for each item) in the CHARLS. Participants with a score of ≥ 12 in the CESD-10 were regarded as having depressive symptoms.26,27
Other potential confounders were body mass index (kg/m2; continuous), and glycosylated hemoglobin (HbA1c) was measured using high-performance liquid chromatography on a Bio-Rad Variant II Turbo. Cholesterol, high-density lipoprotein (HDL-C), low-density lipoprotein (LDL-C), and triglycerides were measured by direct enzymatic methods (Konelab, Thermo Fisher Scientific, Waltham, MA), self-reported dyslipidemia, cancer or malignant tumor, chronic lung diseases, liver disease, heart problems, stroke, kidney disease, stomach or other digestive disease, emotional, nervous, or psychiatric problems, memory-related disease, arthritis or rheumatism, asthma, and diabetes (no/yes).
Statistical Analysis
Categorical variables were described as proportions and continuous variables, as medians and interquartile range, or as means and SD. Comparisons of continuous variables between groups were done using t-tests, one-way ANOVA, or equivalent non-parametric tests.
Generalized linear mixed model (GLMM) regression models with random intercept and slope were used to evaluate longitudinal changes in test performance between study visits 1 to 4.28 The fixed effects (β) and variance components (α) of the generalized mixed linear models were estimated using maximum restricted likelihood methods. The pool function implements the rules for combining the separate estimates and SEs from each of the imputed datasets to provide an overall estimate with SEs, CIs, and p values.29 Subgroup analyses were performed using stratified GLMM.
Missing values for categorical covariates were assigned as a single category. Missing values of quantitative data were not replaced because GLMM can accommodate missing data under the assumption that they are missing at random.
Univariate analyses and the generalized mixed model were done using SPSS (version 26.0), and all p were 2-sided with statistical significance set at <0.05.
Results
Baseline Characteristics
Of 4824 participants included, 61.6% were female, mean age 68.3 (SD=9.5) years at visit 1. There was no significant difference in sex, duration of sleep, HbA1c, LDL-C, depression symptoms, pain, residence, and physically active between hypertensive participants and controls. Non-hypertensive participants had higher levels of current smokers and physically active than hypertensive participants (Table 1).
Age at Diagnosis of Hypertension and Cognitive Function
As shown in Table 2, compared with participants without hypertension, middle-age hypertension was associated with a sharper decline in the memory test (β (95% CI, −1.117 [−1.405, −0.83]), orientation test (β (95% CI, −1.273 [−1.348,−1.198])and global cognitive score (β (95% CI, −1.611 [−1.744, −1.478]), and late hypertension with a reduction in orientation test (β (95% CI, −1.015 [−1.095,−0.936])and global cognitive score (β (95% CI, −1.026 [−1.167, −0.886]). Longer duration of hypertension were independently associated with worse memory scores (β (95% CI, −0.069 [−0.113 to −0.025]).
Besides, hypertensive participants were categorized into their age at diagnosis: < 35 years, 35 to 45 years, 45 to 54 years, 55 to 64 years, and ≥ 65 years. Rate of cognitive decline accelerated with younger age at diagnosis when age was less than 55 years. However, when diagnosis age is higher than 55, rate of cognitive decline slowed down. Individuals with hypertension diagnosed at < 35 (β (95% CI, −1.565 [−1.655, −1.475]), 35 to 44 (β (95% CI, −1.114 [−1.194, −1.034]), 45 to 54 (β (95% CI, −1.036 [−1.118, −0.953]), 55 to 64 (β (95% CI, −0.908 [−1.025, −0.792]), and ≥ 65 years (β (95% CI, −1.105 [−1.341, −0.868]) had lower scores compared with the controls in orientation tests. And individuals with hypertension diagnosed at < 35 (β (95% CI, −2.131 [−2.290, −1.972]), 35 to 44 (β (95% CI, −1.307 [−1.448, −1.166]), 45 to 54 (β (95% CI, −1.065 [−1.210, −0.92]), 55 to 64 (β (95% CI, −0.742 [−0.929, −0.518]) and ≥ 65 years (β (95% CI, −1.448 [−1.865, −1.031]) in global cognitive score (shown in Table S1).
Hypertension Control and Cognitive Function
As depicted in Table 3, uncontrolled hypertension was an independent predictor of greater decline in memory test (β (95% CI, −0.659 [−0.939, −0.380]), orientation test (β (95% CI, −0.259 [−0.365, −0.153]), and global cognitive score (β (95% CI, −0.124 [−0.162, −0.086]) in compared to controlled hypertension. Furthermore, among treated hypertensive individuals, types of antihypertension drugs were not associated with longitudinal changes in cognitive performance (Table 4).
Subgroup Analysis
As shown in Table S2~5, the stratified analysis revealed a highly consistent pattern. Regardless of subgroup, the effect size of the association between age at diagnosis of hypertension and cognitive was stable. In the terms of female, middle-age hypertension was associated with a decline in the memory test (β (95% CI, −2.163 [−2.682, −1.697]), executive function (β (95% CI, 0.234 [0.017, 0.45]), orientation test (β (95% CI, −2.163 [−2.682, −1.697]), and global cognitive score (β (95% CI, −2.144 [−2.357, −1.931]), and late hypertension with a decline in the memory test (β (95% CI, −0.965 [−1.45, −0.48]), orientation test (β (95% CI, −1.355 [−1.484, −1.226]), and global cognitive score (β (95% CI, −1.444 [−1.665, −1.222]), while in male group, middle-age hypertension was associated with a decline in the memory test (β (95% CI, −0.651 [−1.053, −0.249]), executive function (β (95% CI, −0.169 [−0.362, −0.025]), orientation test (β (95% CI, −0.973 [−1.074, −0.872]), and global cognitive score (β (95% CI, −1.259 [−1.448, −1.070]), and late hypertension with a decline in the executive function (β (95% CI, −0.188 [−0.369, −0.007]), orientation test (β (95% CI, −0.837 [−0.944, −0.729]) and global cognitive score (β (95% CI, −0.865 [−1.066, −0.664]).
Discussion
In this large cohort of middle-aged and older adults, hypertension in middle age predicted a reduction in the memory test, orientation test, and global cognitive score. Besides, duration of exposure to hypertension was associated with a decrease in the memory test. Individuals with uncontrolled hypertension had a sharper decline in the memory test, orientation test, and global cognitive score compared with the controlled subjects. Among treated hypertensive individuals, types of antihypertension drugs were not associated with longitudinal changes in cognitive performance. A stronger relationship between age at diagnosis of hypertension and cognitive was detected in female, elementary school or below, depression, living alone, and alcohol consumption.
This work utilized an extended model approach to adjust the potential confounders and performed subgroup and interaction analyses to ensure a stable relationship between age at diagnosis of hypertension and cognitive. Similar to previous findings,13,30,31 our research shows that hypertension in middle age predicted a reduction in memory test, orientation test, and global cognitive score compared to the older age. Besides, hypertension has been associated with virtually all domains of cognitive function, but existing evidence suggest that hypertension does not equally affect distinct cognitive abilities over time.8,32,33 This consistency further confirms the reliability and generality of our results. We found no association between hypertension and executive function, one of the most vulnerable domains to hypertension effects. However, a large cohort by De Menezes et al.17 indicate only modest effects of hypertension at middle age on the memory decline. This discrepancy may be because their study population is much younger than ours, has a higher level of education, and has been followed up for a relatively short time. The high schooling of their cohort, due to mechanisms involving greater cognitive reserve, may contribute to better initial cognitive performance or delayed cognitive decline in the follow-up period,34 hindering the ability to detect a more pronounced modifying effect of age of hypertension onset on cognitive decline in distinct abilities.
Because earlier age of hypertension onset generally implies a longer duration of exposure to hypertension, we analyzed these 2 components separately. Our results demonstrated an adverse effect of increased duration of hypertension on memory tests in the follow-up period. However, our results do not support an adverse effect of increased duration of hypertension on global cognitive performance, as reported by some studies.35,36 The lack of association between duration of hypertension and global cognitive performance in our study is consistent with our findings about age of hypertension diagnosis: greater effect of hypertension at an older age hypertension at middle age in cognitive performance over 8-year follow-up.
Our findings help to further elucidate the potential benefits of blood pressure control on cognitive function, as memory, orientation, and global scores declined significantly faster in the uncontrolled hypertensive group than in the control group, in line with recently published finding.37 Contrariwise, in our analysis, types of antihypertension drugs were not associated with longitudinal changes in cognitive performance. A randomized, multicenter, double-blind, noninferiority trial showed that the Songling Xuemaikang capsule—a Chinese herbal formula—was effective for essential hypertension,38 which is inconsistent with our research. However, medical records were not available in CHARLS; thus, the use of self-reported measures may also have some degree of bias.
Our study has several limitations; we cannot rule out misclassifications of age of hypertension onset since it was self-reported by hypertensive individuals at baseline. Furthermore, our study population is relatively old; a potential cognitive benefit of higher BP levels in the elderly due to better brain perfusion, often present as a result of decompensated autoregulatory brain mechanisms, may hinder the analysis of the effect of hypertension onset at different ages on cognitive performance.35,39 CHARLS did not perform a comprehensive screening to rule out cognitive impairment at entry, and stroke information was self-reported. In addition, our results cannot be extrapolated to unevaluated domains and abilities. Finally, we did not evaluate the causes of inadequate control of hypertension in CHARLS: low adherence to treatment, more severe form of hypertension, or more advanced stage of the disease. Although important, such analysis is beyond the scope of this study.
Data Availability
The data used in the study are accessible to be downloaded publicly at https://charls.charlsdata.com/pages/data/111/zh-cn.html
References
Mills KT, Bundy JD, Kelly TN, et al. Global disparities of hypertension prevalence and control: a systematic analysis of population-based studies from 90 countries. Circulation. 2016;134(6):441-450.
Lu J, Lu Y, Wang X, et al. Prevalence, awareness, treatment, and control of hypertension in China: data from 1· 7 million adults in a population-based screening study (China PEACE Million Persons Project). The Lancet. 2017;390(10112):2549-2558.
Okusaga O, Stewart MC, Butcher I, Deary I, Fowkes FGR, Price JF. Smoking, hypercholesterolaemia and hypertension as risk factors for cognitive impairment in older adults. Age Ageing. 2013;42(3):306-311.
Uiterwijk R, Huijts M, Staals J, et al. Subjective cognitive failures in patients with hypertension are related to cognitive performance and cerebral microbleeds. Hypertension. 2014;64(3):653-657.
Walker KA, Power MC, Gottesman RF. Defining the relationship between hypertension, cognitive decline, and dementia: a review. Curr Hypertens Rep. 2017;19(3):1-16.
van Eersel ME, Joosten H, Gansevoort R, Slaets J, Izaks G. Treatable vascular risk and cognitive performance in persons aged 35 years or older: longitudinal study of six years. J Prev Alzheimer's Dis. 2019;6(1):42-49.
Xue H, Hou P, Li Y, Mao Xe, Wu L, Liu Y. Factors for predicting reversion from mild cognitive impairment to normal cognition: a meta-analysis. Int J Geriatr Psychiatry. 2019;34(10):1361-1368.
Debette S, Seshadri S, Beiser A, et al. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology. 2011;77(5):461-468.
Yaffe K, Vittinghoff E, Pletcher MJ, et al. Early adult to midlife cardiovascular risk factors and cognitive function. Circulation. 2014;129(15):1560-1567.
Suvila K, Lima JA, Yano Y, Tan ZS, Cheng S, Niiranen TJ. Early-but not late-onset hypertension is related to midlife cognitive function. Hypertension. 2021;77(3):972-979.
Rovio SP, Pahkala K, Nevalainen J, et al. Cardiovascular risk factors from childhood and midlife cognitive performance: the Young Finns Study. J Am Coll Cardiol. 2017;69(18):2279-2289.
Walker KA, Sharrett AR, Wu A, et al. Association of midlife to late-life blood pressure patterns with incident dementia. JAMA. 2019;322(6):535-545.
Gottesman RF, Schneider AL, Albert M, et al. Midlife hypertension and 20-year cognitive change: the atherosclerosis risk in communities neurocognitive study. JAMA Neurol. 2014;71(10):1218-1227.
Köhler S, Baars MA, Spauwen P, Schievink S, Verhey FR, van Boxtel MJ. Temporal evolution of cognitive changes in incident hypertension: prospective cohort study across the adult age span. Hypertension. 2014;63(2):245-251.
Abell JG, Kivimäki M, Dugravot A, et al. Association between systolic blood pressure and dementia in the Whitehall II cohort study: role of age, duration, and threshold used to define hypertension. Eur Heart J. 2018;39(33):3119-3125.
Power MC, Tchetgen EJ, Sparrow D, Schwartz J, Weisskopf MG. Blood pressure and cognition: factors that may account for their inconsistent association. Epidemiology. 2013;24(6):886-893.
de Menezes ST, Giatti L, Brant LCC, et al. Hypertension, Prehypertension, and Hypertension Control: Association With Decline in Cognitive Performance in the ELSA-Brasil Cohort. Hypertension. 2021;77(2):672-681.
Wei J, Yin X, Liu Q, Tan L, Jia C. Association between hypertension and cognitive function: A cross-sectional study in people over 45 years old in China. J Clin Hypertens (Greenwich, Conn). 2018;20(11):1575-1583.
Ma Y, Hua R, Yang Z, Zhong B, Yan L, Xie W. Different hypertension thresholds and cognitive decline: a pooled analysis of three ageing cohorts. BMC Med. 2021;19(1):287.
Song H, Feng D, Wang R, et al. Urban-rural disparity in the utilization of national community-based hypertension monitoring service-results from the China Health and Retirement Longitudinal Study, 2015. PeerJ. 2019;7:e7842.
Zhang B, Jiang S. Heterogeneity in longitudinal trajectories of cognitive performance among middle-aged and older individuals with hypertension: Growth mixture modeling across an 8-year cohort study. Hypertens Res. 2021.
Xu H, Zhang Z, Li L, Liu J. Early life exposure to China's 1959-61 famine and midlife cognition. Int J Epidemiol. 2018;47(1):109-120.
Dregan A, Stewart R, Gulliford MC. Cardiovascular risk factors and cognitive decline in adults aged 50 and over: a population-based cohort study. Age Ageing. 2013;42(3):338-345.
Zheng F, Yan L, Yang Z, Zhong B, Xie W. HbA(1c), diabetes and cognitive decline: the English Longitudinal Study of Ageing. Diabetologia. 2018;61(4):839-848.
Xie W, Zheng F, Yan L, Zhong B. Cognitive Decline Before and After Incident Coronary Events. J Am Coll Cardiol. 2019;73(24):3041-3050.
Zheng F, Zhong B, Song X, Xie W. Persistent depressive symptoms and cognitive decline in older adults. Br J Psychiatry. 2018;213(5):638-644.
Li C, Miles T, Shen L, et al. Early-life exposure to severe famine and subsequent risk of depressive symptoms in late adulthood: the China Health and Retirement Longitudinal Study. Br J Psychiatry. 2018;213(4):579-586.
Cnaan A, Laird NM, Slasor P. Using the general linear mixed model to analyse unbalanced repeated measures and longitudinal data. Stat Med. 1997;16(20):2349-2380.
Rubin DB. Multiple imputation for survey nonresponse. In: New York: Wiley; 1987.
Power MC, Tingle JV, Reid RI, et al. Midlife and late-life vascular risk factors and white matter microstructural integrity: the atherosclerosis risk in communities neurocognitive study. J Am Heart Assoc. 2017;6(5):e005608.
Ninomiya T, Ohara T, Hirakawa Y, et al. Midlife and late-life blood pressure and dementia in Japanese elderly: the Hisayama study. Hypertension. 2011;58(1):22-28.
Waldstein SR. The relation of hypertension to cognitive function. Curr Dir Psychol Sci. 2003;12(1):9-12.
Waldstein SR, Manuck SB, Ryan CM, Muldoon MF. Neuropsychological correlates of hypertension: review and methodologic considerations. Psychol Bull. 1991;110(3):451.
Stern Y. Cognitive reserve. Neuropsychologia. 2009;47(10):2015-2028.
Power MC, Tchetgen EJT, Sparrow D, Schwartz J, Weisskopf MG. Blood pressure and cognition: factors that may account for their inconsistent association. Epidemiology (Cambridge, Mass). 2013;24(6):886-93.
Köhler S, Baars MA, Spauwen P, Schievink S, Verhey FR, van Boxtel MJ. Temporal evolution of cognitive changes in incident hypertension: prospective cohort study across the adult age span. Hypertension. 2014;63(2):245-251.
Levine DA, Galecki AT, Langa KM, et al. Blood Pressure and Cognitive Decline Over 8 Years in Middle-Aged and Older Black and White Americans. Hypertension. 2019;73(2):310-318.
Lai X, Dong Z, Wu S, et al. Efficacy and Safety of Chinese Herbal Medicine Compared With Losartan for Mild Essential Hypertension: A Randomized, Multicenter, Double-Blind, Noninferiority Trial. Circ Cardiovasc Qual Outcomes. 2022;15(3):e007923.
Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol. 2005;4(8):487-499.
Acknowledgements
We thank L. Ding and X. Zhu designed research; L. Ding and X. Zhang analyzed the data; L. Ding wrote the manuscript; Z. Xiong and F. Yang provided language help, and X. Zhu had primary responsibility for the final content. All authors revised it critically for important intellectual content. Acknowledgments are made to National Natural Science Foundation of China (82003448) and Fund of Hubei Provincial Education Department (Q20202010) for partial support of this research.
Funding
This study was supported by National Natural Science Foundation of China (82003448) and Fund of Hubei Provincial Education Department (Q20202010) to Xinhong Zhu.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics Approval
The CHARLS were approved by the Peking University Institutional Review Board, respectively. All participants provided written informed consent.
Ethics Approval
The ethical approval of data collection was from the Biomedical Ethics Review Committee of Peking University (IRB00001052–11015). Every participant signed an informed consent before investigation, and their information were kept anonymous.
Conflict of Interest
The authors declare no conflict interests.
Permission to Reproduce Materials from Other Sources
No
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 49 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ding, L., Zhu, X., Xiong, Z. et al. The Association of Age at Diagnosis of Hypertension with Cognitive Decline: the China Health and Retirement Longitudinal Study (CHARLS). J GEN INTERN MED 38, 1431–1438 (2023). https://doi.org/10.1007/s11606-022-07951-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11606-022-07951-1