ABSTRACT
BACKGROUND
Minorities have lower adherence to cardiovascular medications and have worst cardiovascular outcomes post coronary stent placement
OBJECTIVE
The aim of this study is to compare the efficacy of phone-delivered Motivational Interviewing (MINT) to an educational video at improving adherence to antiplatelet medications among insured minorities.
DESIGN
This was a randomized study.
PARTICIPANTS
We identified minorities with a recently placed coronary stent from an administrative data set by using a previously validated algorithm.
INTERVENTIONS
MINT subjects received quarterly phone calls and the DVD group received a one-time mailed video.
MAIN MEASURES
Outcome variables were collected at baseline and at 12-month post-stent, using surveys and administrative data. The primary outcome was antiplatelet (clopidogrel and prasugrel) adherence measured by Medication Possession Ratio (MPR) and self- reported adherence (Morisky score). We also measured appropriate adherence defined as an MPR ≥ 0.80.
KEY RESULTS
We recruited 452 minority subjects with a new coronary stent (44 % Hispanics and 56 % Black). The patients had a mean age of 69.5 ± 8.8, 58 % were males, 78 % had an income lower than $30,000 per year and only 22 % had achieved high school education or higher. The MPR for antiplatelet medications was 0.77 for the MINT group compared to 0.70 for the DVD group (p < 0.05). The percentage of subjects with adequate adherence to their antiplatelet medication was 64 % in the MINT group and 50 % in the DVD group (p < 0.01). Self-reported adherence at 12 months was higher in the MINT group compared to the DVD group (p < 0.01). Results were similar among drug-eluting stent (DES) recipients.
CONCLUSIONS
Among racial minorities, a phone-based motivational interview is effective at improving adherence to antiplatelet medications post coronary stent placement. Phone-based MINT seems to be a promising and cost-effective strategy to modify risk behaviors among minority populations at high cardiovascular risk.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
BACKGROUND
The safe use of coronary stents requires the daily use of thienopyridines for 3 to 12 months after percutaneous coronary intervention (PCI).1 The premature discontinuation of these medications1 is associated with an increased risk of in-stent thrombosis, cardiovascular events and death.2 – 6 Unfortunately, lack of medication adherence is common and is associated with significant health care costs and mortality.7 , 8 Vulnerable groups such as racial minorities and the elderly have a higher risk of poor adherence.5 , 6 , 9 , 10 In the case of antiplateletet medications, blacks are more likely to not receive a clopidogrel prescription11 or to not fill it after PCI. Further, our prior analyses of insurer claims data showed that minority populations were less adherent to antiplatelet medications post drug-eluting stent (DES) placement.12 The lack of appropriate use of evidence-based therapies after PCI may partially contribute to the excess morbidity and mortality reported among minorities.13 , 14 , 6
The improvement of medication adherence is a global priority,15 yet only a few experimental interventions have been proven to be successful.16 These include interventions that reduce out-of-pocket costs, simplify regimens, facilitate delivery of medications, or offer behavioral support.16 – 19 Unfortunately, many of these approaches have been difficult to sustain outside of research settings.16 To test potentially sustainable interventions in real world settings, we conducted a randomized controlled clinical trial (RCT) testing the comparative effectiveness of a telephone-based behavioral intervention versus an educational DVD at improving 12-month adherence to thienopiridines among minority coronary stent recipients.
METHODS
Study Design
We conducted a randomized clinical trial of balanced, parallel groups that included members of a health benefits company (Humana, Inc.) across 22 states in the United States. We compared two interventions of different complexity: a phone-based motivational interview and a mailed educational DVD; the group receiving the DVD was considered the less intense comparison group. All subjects continued to receive all usual health care by their usual providers, and the investigative team had no contact with these personnel.
Study Setting
Data from a large health benefits carrier was used to identify subjects who received a coronary stent and who belonged to a racial/ethnic minority group. This carrier had 3.5 million beneficiaries enrolled in a variety of insurance products, including commercial, Medicare and Medicaid plans. Humana’s electronic data warehouse (EDW) merges three distinct files: membership files containing demographic and insurance information; medical files containing up to nine International Classification of Diseases (ICD) codes, current procedural terminology (CPT) codes or diagnosis related group (DRG) codes per encounter; and a pharmacy claims file containing real-time data on dispensed medications (name, dosage and quantity). A prior comparison of this data to nationally representative samples showed that the age-adjusted prevalence of diseases in this commercial insurance database shared similar distributions to nationally representative samples, and had only slightly lower age-adjusted prevalence of major health conditions versus national samples.
Participants
We recruited consecutive subjects age 18 years and older with a medical claim for a drug-eluting stent (DES) or a bare metal stent (BMS) between December 2009 and October of 2010. The positive predictive value of using claims data for identification of patients receiving a stent is 93 % (CI 85–97 %).20 The date of the member’s stent claim was considered the index date.
Inclusion/Exclusion Criteria
Subjects needed to have received a coronary stent in the previous 90 days and to have filled at least one prescription for clopidogrel or prasugrel. To identify eligible minorities, we used Medicare race codes (MRC), geo-coding algorithms and the Census-based Spanish surname list21 – 23 to identify subjects having a high probability of being Hispanic or non-Hispanic blacks. The accuracy of this algorithm was 91 % for the identification of black subjects and 92 % for Hispanic subjects.20 We then verified race/ethnicity by self-report during the recruitment calls. We excluded subjects who had cognitive impairments that prevented them from participating in the phone encounters, as well as subjects who reported they were likely to dis-enroll from their insurer during the next year.
Recruitment Procedures
Our sample size calculations showed that with 217 subjects in each arm, we would have 93 % power using a two-tailed test to detect a 15 percentage-point difference in improvement of medication adherence from a baseline of 50 % between the comparison groups. To recruit these subjects, we conducted monthly queries of the claims data to identify potentially eligible subjects. The study coordinator sent recruitment letters to all eligible subjects. This letter included an opt-out option. Subjects identified as likely to be Hispanic received the letter in English and Spanish and were contacted by a bilingual recruiter. One week after the letters were sent, we began recruitment calls. We conducted ten call attempts during a 2-week period and another five attempts after a 1-week waiting period. Once subjects were reached by phone, we screened for eligibility based on stent placement date and on being a minority subject.
After obtaining informed consent, the recruiter completed a baseline questionnaire. We stratified subjects according to race/ethnicity and randomized them to either a phone-based motivational interview intervention or a mailed role-modeling DVD. We used a block randomization strategy with a block size of six and a 1:1 ratio stratified by race/ethnicity. At 12 months after the index date, a phone-based questionnaire was repeated. The study was approved by the University of Miami Institutional Review Board. Due to an oversight, registration in clinicaltrials.gov was delayed and occurred retrospectively. There were only two minor logistical changes to the original study protocol and all the outcomes were the same as those originally proposed to the National Institutes of Health (NIH).
INTERVENTIONS
Motivational Interviewing (MINT) is a patient-centered, directive method for enhancing intrinsic motivation to change by exploring and resolving the ambivalence subjects feel with respect of health choices. MINT delivered in person has been successful at reducing weight; achieving smoking cessation; improving cardiovascular risk profile;24 – 27 and improving adherence, particularly to HIV medications.18 , 28 – 30 For the study, we trained two Humana nurses in the delivery of MINT. Training consisted of a 3-day workshop conducted by an experienced MINT trainer and by a specialist in the MINT Integrity Coding system (MITI). Nurses also received a 1-week training on coronary artery disease, antiplatelet medications, known predictors of adherence, and study-specific protocols and data collection procedures.
In the study, nurses delivered a MINT call every 3 months (four calls in a period of 12 months) to elicit individual values, preferences, arguments for change, reasons for past failures, and to empower patients to resolve ambivalence and develop a behavior modification plan. The first call was to establish rapport with patients, communicate the call agenda while emphasizing patients’ personal choice, and to foster equal power sharing. This call also included a review of study procedures, open-ended questions on patients’ typical day since stent placement, assessment of health goals and motivation/ confidence in the ability to change behaviors, assessment of current stressor/s, and administration of the Morisky Medication Adherence Scale (MMAS). Subsequent phone calls were less structured, but the MMAS was administered in all calls to initiate a dialogue regarding patients’ concerns around medication adherence. To assess study fidelity, a MINT specialist (LHF) audited 10 % of randomly selected phone calls and used MITI to code for the degree of empathy, direction, and MINT techniques employed by the nurses. The MINT specialist met once per month with the nursing supervisor to provide feedback on the calls and make recommendations about competencies that needed development.
For ethical reasons, rather than a standard control group, we chose a mailed DVD as the comparison group. This ensured that all patients had at least some information on the need to use thienopiridines after stent placement. The study team used role theory and social cognitive theory principles to design and produce the video. Subjects randomized to the DVD received a one-time mailed DVD featuring two patients (one of each race/ethnicity) modeling appropriate coronary artery disease role-taking behavior and discussing their experiences with the stent procedure and with the antiplatelet medications. The DVD also included a cardiologist discussing the important aspects that patients need to know after stent placement. Spanish-speaking subjects were mailed a Spanish version of the DVD. Subjects randomized to this arm were contacted by phone at the beginning and end of the study. We also mailed subjects quarterly reminders with secondary prevention tips and requesting updated contact information.
OUTCOME MEASURES
Primary Endpoint: Adherence to Antiplatelet Medications
Our primary measure of adherence was the medication possession ratio (MPR). This claims-based measure has been shown to have improved accuracy and have higher correlation with biological markers of adherence than pill counts.31 – 33 It also reduces recall bias that may occur with self-reported adherence measures. To obtain the MPR, we used pharmacy claims data for each subject that were obtained 6 months after all subjects had completed the study. We calculated the MPR as the sum of the days’ supply of antiplatelet medications divided by 365 days.34 We considered adherence as appropriate based on a MPR ≥ 0.80.33 , 35 This cutoff corresponds to the subject having medication available 80 % or more during the 12 month follow-up period.
Secondary End Points and Other Covariates
Our secondary end points wer self-reported medication adherence using the four-item Morisky Medication Adherence Scale (MMAS-4).36 The MMAS-4 is a generic self-reported, medication-taking behavior scale in which a specific health issue is inserted as the “health concern” in each of the questions. The MMAS consists of four items with a scoring scheme of “Yes” = 0 and “No” = 1 (Table 1). The items are summed to give a range of scores from 0 to 4.
Additional exploratory end points included hospitalization for myocardial infarction (MI) up to 12 months after the index stent and all-cause mortality. We classified subjects as having a MI during the follow-up period if they had a discharge with the ICD-9 for acute MI in primary or secondary position and an associated hospitalization with a length of stay of 3 days or more.37 For mortality, we used the National Death Index.38 Additional outcomes included self-reported forgetfulness when taking antiplatelet medications and completion of therapy.
During the baseline survey, we also collected data on additional covariates, including age, gender, marital status, income, education level, insurance, smoking history, health literacy,39 type of stent placed and depression (PHQ-9).40 We used claims data to ascertain insurance type, charlson comorbidity score,41 , 42 and if subjects presented with an MI at the time of the index stent.
STATISTICAL ANALYSES
Our primary analysis examined the proportion of subjects that reached an adequate MPR of ≥ 0.80, using chi-square comparisons and t-tests to examine the mean MPR between groups, both based on the intent to treat principle. To examine differences in self-reported adherence, we used the Wilcoxon Rank Sum Test (MMAS scores were not normally distributed). In addition, we calculated the difference between the baseline and 12-month score for each group and used a t-test with unequal variances assumptions to compare the pre-post difference between the intervention groups. We used chi-square to compare to all other secondary outcomes.
All analyses were performed using SAS 9.0 (Cary, North Carolina), and all significance tests were two-tailed.
RESULTS
Overall Sample Characteristics
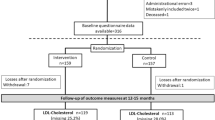
We recruited 452 subjects, of which 44 % were Hispanic and 56 % black. We randomized 227 to MINT and 225 to the mailed video. Subjects had a mean age of 69.5 years ±8.8, 58 % were male, 78 % had annual incomes < $30,000 and only 22 % had completed high school. Most subjects, 84 %, received a drug-eluting stent (Table 2). At 12 months post index stent placement, we had pharmacy claims data available on 422 subjects (dropout rate of 6.1 % in MINT and 7.1 % in DVD) (Fig. 1).
Primary Outcome
We found that the percentage of subjects with adequate adherence to their antiplatelet medication was 64 % among subjects in the MINT group and 50 % in the DVD group (p < 0.01). The 12-month mean MPR was 0.77 for the MI group versus 0.70 for the DVD group (p < 0.05) (Table 3). Analyses restricted to those with a DES showed similar results, with 65 % of MINT subjects achieving appropriate adherence versus 50 % of DVD recipients (Table 4). Although only 51 % subjects in the DVD reported actually watching the DVD, the mean MPR was similar among those who watched and did not watch the DVD (0.71 versus 0.70, p > 0.05).
Secondary Outcomes
At 12 months, we were able to conduct interviews among 74 % of subjects randomized to the MINT intervention group and 76 % of those in the DVD group. Among these, self-reported adherence scores were higher in the MINT group compared to the DVD group (3.82 versus 3.60, p < 0.01). (Table 3) Only 8 % of subjects in MINT reported being forgetful about taking medications compared to 24 % in the DVD group (p < 0.01) (Table 3). In addition, in pre-post comparisons, self-reported adherence was significantly improved from baseline to 12 months among MINT recipients (p < 0.01) and did not change among the DVD recipients.
Claims data showed that 41 subjects in the study experienced an MI and there were 12 deaths (based on death index). By group, 11 % in the MINT group and 8 % in the DVD group experienced an MI and 2 % in the MINT group and 5 % in the DVD group died. However, our sample size was not adequately powered for these comparisons and these are considered as hypothesis-generating findings.
DISCUSSION
Among a cohort of black and Hispanic patients, we found that compared to an educational video, phone-based motivational interviews significantly improved adherence to antiplatelet medications post stent placement. Several other studies,18 , 43 , 28 but not all,44 have also shown that MINT is successful at improving medication adherence. The current study extends this literature by showing that a phone-based approach is effective among a large, geographically dispersed sample of minority patients whom had recently undergone a major cardiovascular procedure. This suggests that phone-based MINT is a feasible and potentially cost-effective strategy to increase medication adherence among typically hard-to-reach, vulnerable populations.
The etiology of mediation non-adherence is complex and has many root causes.45 These include limited access to health care, socio-economic status, co-payments, polypharmacy, cultural competency, trust and belief systems. Many of these barriers will need to be addressed within the context of major policy and health delivery changes. Yet, we found that MINT offered a feasible and efficacious approach, in which subjects were encouraged to find solutions to their own adherence barriers. A counselor who was not part of the patient’s health care delivery system delivered the intervention telephonically. This approach may facilitate the implementation and sustainability of this intervention in real world settings. The possibility that some patients may be more open to discussing their individual circumstances with independent counselors by phone deserves further study. Similarly, timing and condition targeting may influence the readiness and ultimate response to MINT. Most of the in-person MINT interventions that have succeeded recruited subjects at critical therapeutic times.46 – 48
Our randomized trial study had several strengths, including a large cohort of minority subjects across several states and the use of both an objective as well as self-reported measure of medication adherence. While many of our patients had low incomes and educational attainment, they were all insured. This limits the generalizability of our findings, particularly for uninsured populations that face greater access-to-care barriers. Second, we chose an active comparison group. Yet, only half the subjects watched the DVD and despite a strong conceptual basis for the design of the DVD, adherence rates were similar among who those watched the DVD and those who did not. Unfortunately, other studies have also shown that simple educational interventions may not be sufficient to modify adherence behavior.49 – 51
Lastly, we examined adherence to antiplatelet therapy at 12 months, since that is standard recommendation after a DES. However, expert opinion and guidelines, as well as the appropriate length of use of such medications continue to evolve.52
CONCLUSIONS
We showed that a phone-based MINT delivered remotely by interventionists outside of the clinic setting is effective at improving adherence to antiplatelet medications post coronary stent placement in a minority population. Among the many features of the Affordable Care Act, it prioritizes patient-centered, cost-effective strategies. Similarly, health insurers and large care delivery systems are also likely to be very interested in evidence-based strategies offering simple, scalable, yet individualized approaches to improving adherence to chronic cardiovascular medications. Phone-based MINT is one strategy that can be added to any health system with minimal additional changes required. The impact of this strategy on health outcomes and the cost effectiveness of this approach are areas that warrant future investigation.
REFERENCES
Grines C. Prevention of premature discontinuation of dual antiplatelet therapy in patients with coronary artery stents: a science advisory from the American Heart Association, American College of Cardiology, Society for Cardiovascular Angiography and Interventions, American College of Surgeons, and American Dental Association, with representation from the American College of Physicians. Circulation (New York, N.Y.). 2007;115(6):813–8.
Spertus JA, Kettelkamp R, Vance C, et al. Prevalence, predictors, and outcomes of premature discontinuation of thienopyridine therapy after drug-eluting stent placement: results from the PREMIER registry. Circulation. 2006;113(24):2803–9.
Iakovou I, Schmidt T, Bonizzoni E, et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA. 2005;293(17):2126–30.
Petersen JL, Barron JJ, Hammill BG, et al. Clopidogrel use and clinical events after drug-eluting stent implantation: findings from the HealthCore integrated research database. Am Heart J. 2010;159(3):462–470.e1.
Brar SS, ten Berg J, Marcucci R, et al. Impact of platelet reactivity on clinical outcomes after percutaneous coronary intervention. A collaborative meta-analysis of individual participant data. J Am Coll Cardiol. 2011;58(19):1945–54.
Roth GA, Morden NE, Zhou W, Malenka DJ, Skinner J. Clopidogrel use and early outcomes among older patients receiving a drug-eluting coronary artery stent. Circ Cardiovasc Qual Outcomes. 2012;5(1):103–12.
World Health Organization. Noncommunicable diseases and mental health: Progress report 2002–2003. Geneva:; 2003.
Chowdhury R, Khan H, Heydon E, et al. Adherence to cardiovascular therapy: a meta-analysis of prevalence and clinical consequences. Eur Heart J. 2013;34(38):2940–8.
Vik SA, Maxwell CJ, Hogan DB. Measurement, correlates, and health outcomes of medication adherence among seniors. Ann Pharmacother. 2004;38(2):303–12.
Rodriguez F, Cannon CP, Steg PG, et al. Predictors of long-term adherence to evidence-based cardiovascular disease medications in outpatients with stable atherothrombotic disease: findings from the REACH registry. Clin Cardiol. 2013;36(12):721–7.
Sonel AF, Good CB, Mulgund J, et al. Racial variations in treatment and outcomes of black and white patients with high-risk non-ST-elevation acute coronary syndromes: insights from CRUSADE (can rapid risk stratification of unstable angina patients suppress adverse outcomes with early implementation of the ACC/AHA guidelines?). Circulation. 2005;111(10):1225–32.
Palacio AM, De Marchena E, Parris D, Li H, Florez H, O’Neill W. Impact of lack of clopidogrel use in stent related myocardial infarction. Journal of the American College of Cardiology. 2008;51:236–70.
Kumar RS, Douglas PS, Peterson ED, et al. Effect of race and ethnicity on outcomes with drug-eluting and bare metal stents: results in 423 965 patients in the linked national cardiovascular data registry and centers for medicare & medicaid services payer databases. Circulation. 2013;127(13):1395–403.
Kumbhani DJ, Steg PG, Cannon CP, et al. Adherence to secondary prevention medications and four-year outcomes in outpatients with atherosclerosis. Am J Med. 2013;126(8):693–700.e1.
World Health Organization. Adherence to long term therapies:Evidence for action. 2003.
Haynes RB, Yao X, Degani A, Kripalani S, Garg A, McDonald HP. Interventions to enhance medication adherence. Cochrane Database Syst Rev. 2005(4):CD000011.
Boland GM, Chang GJ, Haynes AB, et al. Association between adherence to national comprehensive cancer network treatment guidelines and improved survival in patients with colon cancer. Cancer. 2013;119(8):1593–601.
Easthall C, Song F, Bhattacharya D. A meta-analysis of cognitive-based behaviour change techniques as interventions to improve medication adherence. BMJ Open. 2013;3(8): doi:10.1136/bmjopen,2013-002749.
Viswanathan M, Golin CE, Jones CD, et al. Interventions to improve adherence to self-administered medications for chronic diseases in the united states: a systematic review. Ann Intern Med. 2012;157(11):785–95.
Palacio AM, Tamariz LJ, Uribe C, et al. Can claims-based data be used to recruit black and hispanic subjects into clinical trials? Health Serv Res. 2011.
Wei II, Virnig BA, John DA, Morgan RO. Using a spanish surname match to improve identification of hispanic women in medicare administrative data. Health Serv Res. 2006;41(4 Pt 1):1469–81.
Elliott MN. Using the census Bureau’s surname list to improve estimates of race/ethnicity and associated disparities. Health Serv Outcomes Res. 2009;9(2):69–83.
Fiscella K, Fremont AM. Use of geocoding and surname analysis to estimate race and ethnicity. Health Serv Res. 2006;41(4 Pt 1):1482–500.
Armstrong MJ, Mottershead TA, Ronksley PE, Sigal RJ, Campbell TS, Hemmelgarn BR. Motivational interviewing to improve weight loss in overweight and/or obese patients: a systematic review and meta-analysis of randomized controlled trials. Obes Rev. 2011;12(9):709–23.
Hettema JE, Hendricks PS. Motivational interviewing for smoking cessation: a meta-analytic review. J Consult Clin Psychol. 2010;78(6):868–84.
Heckman CJ, Egleston BL, Hofmann MT. Efficacy of motivational interviewing for smoking cessation: a systematic review and meta-analysis. Tob Control. 2010;19(5):410–6.
Thompson DR, Chair SY, Chan SW, Astin F, Davidson PM, Ski CF. Motivational interviewing: a useful approach to improving cardiovascular health? J Clin Nurs. 2011;20(9–10):1236–44.
Ogedegbe G, Chaplin W, Schoenthaler A, et al. A practice-based trial of motivational interviewing and adherence in hypertensive African Americans. Am J Hypertens. 2008;21(10):1137–43.
Pradier C, Bentz L, Spire B, et al. Efficacy of an educational and counseling intervention on adherence to highly active antiretroviral therapy: French prospective controlled study. HIV Clin Trials. 2003;4(2):121–31.
Holstad MM, Essien JE, Ekong E, Higgins M, Teplinskiy I, Adewuyi MF. Motivational groups support adherence to antiretroviral therapy and use of risk reduction behaviors in HIV positive nigerian women: a pilot study. Afr J Reprod Health. 2012;16(3):14–27.
Farmer KC. Methods for measuring and monitoring medication regimen adherence in clinical trials and clinical practice. Clin Ther. 1999;21(6):1074–90; discussion 1073.
Grossberg R, Zhang Y, Gross R. A time-to-prescription-refill measure of antiretroviral adherence predicted changes in viral load in HIV. J Clin Epidemiol. 2004;57(10):1107–10.
Steiner JF, Prochazka AV. The assessment of refill compliance using pharmacy records: methods, validity, and applications. J Clin Epidemiol. 1997;50(1):105–16.
Hess LM, Raebel MA, Conner DA, Malone DC. Measurement of adherence in pharmacy administrative databases: a proposal for standard definitions and preferred measures. Ann Pharmacother. 2006;40(7–8):1280–88.
Steiner JF. Rethinking adherence. Ann Intern Med. 2012;157(8):580–5.
Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24(1):67–74.
Kiyota Y, Schneeweiss S, Glynn RJ, Cannuscio CC, Avorn J, Solomon DH. Accuracy of medicare claims-based diagnosis of acute myocardial infarction: estimating positive predictive value on the basis of review of hospital records. Am Heart J. 2004;148(1):99–104.
Fillenbaum GG, Burchett BM, Blazer DG. Identifying a national death index match. Am J Epidemiol. 2009;170(4):515–8.
Chew LD, Griffin JM, Partin MR, et al. Validation of screening questions for limited health literacy in a large VA outpatient population. J Gen Intern Med. 2008;23(5):561–6.
Kroenke K, Spitzer RL, Williams JBW. The PHQ-9. validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–13.
Deyo RA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613.
Needham DM, Scales DC, Laupacis A, Pronovost PJ. A systematic review of the Charlson comorbidity index using Canadian administrative databases: a perspective on risk adjustment in critical care research. J Crit Care. 2005;20(1):12–9.
Rubak S, Sandbaek A, Lauritzen T, Christensen B. Motivational interviewing: a systematic review and meta-analysis. Br J Gen Pract. 2005;55(513):305–12.
Heisler M, Hofer TP, Klamerus ML, et al. Study protocol: the adherence and intensification of medications (AIM) study--a cluster randomized controlled effectiveness study. Trials. 2010;11:95.
Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487–97.
Konkle-Parker DJ, Erlen JA, Dubbert PM, May W. Pilot testing of an HIV medication adherence intervention in a public clinic in the deep south. J Am Acad Nurse Pract. 2012;24(8):488–98.
Golin CE, Earp J, Tien HC, Stewart P, Porter C, Howie L. A 2-arm, randomized, controlled trial of a motivational interviewing-based intervention to improve adherence to antiretroviral therapy (ART) among patients failing or initiating ART. J Acquir Immune Defic Syndr. 2006;42(1):42–51.
Interian A, Lewis-Fernandez R, Gara MA, Escobar JI. A randomized-controlled trial of an intervention to improve antidepressant adherence among latinos with depression. Depress Anxiety. 2013;30(7):688–96.
Clarkesmith DE, Pattison HM, Lip GY, Lane DA. Educational intervention improves anticoagulation control in atrial fibrillation patients: the TREAT randomised trial. PLoS One. 2013;8(9):e74037.
Nover C, Jackson SS. Primary care-based educational interventions to decrease risk factors for metabolic syndrome for adults with major psychotic and/or affective disorders: a systematic review. Syst Rev. 2013;2:116,4053-2-116.
Crouch R, Wilson A, Newbury J. A systematic review of the effectiveness of primary health education or intervention programs in improving rural women’s knowledge of heart disease risk factors and changing lifestyle behaviours. Int J Evid Based Healthc. 2011;9(3):236–45.
Feres F, Costa RA, Abizaid A, et al. Three vs twelve months of dual antiplatelet therapy after zotarolimus-eluting stents: the OPTIMIZE randomized trial. JAMA. 2013;310(23):2510–22.
Conflict of Interest
The authors declare no conflicts of interest.
Funding
This study was sponsored by the National Center on Minority Health and Health Disparities (NCMHD), under Recovery Act (ARRA) grant 5 RC1 MD004327.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Palacio, A.M., Uribe, C., Hazel-Fernandez, L. et al. Can Phone-Based Motivational Interviewing Improve Medication Adherence to Antiplatelet Medications After a Coronary Stent Among Racial Minorities? A Randomized Trial. J GEN INTERN MED 30, 469–475 (2015). https://doi.org/10.1007/s11606-014-3139-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11606-014-3139-8