Abstract
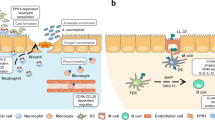
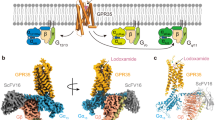
Proton-activated G protein-coupled receptors (GPCRs), initially discovered by Ludwig in 2003, are widely distributed in various tissues. These receptors have been found to modulate the immune system in several inflammatory diseases, including inflammatory bowel disease, atopic dermatitis, and asthma. Proton-activated GPCRs belong to the G protein-coupled receptor family and can detect alternations in extracellular pH. This detection triggers downstream signaling pathways within the cells, ultimately influencing the function of immune cells. In this review, we specifically focused on investigating the immune response of proton-activated GPCRs under inflammatory conditions.
Similar content being viewed by others
References
Ludwig MG, Vanek M, Guerini D, et al. Proton-sensing G-protein-coupled receptors. Nature, 2003,425(6953):93–98
Cai H, Wang X, Zhang Z, et al. Moderate l-lactate administration suppresses adipose tissue macrophage M1 polarization to alleviate obesity-associated insulin resistance. J Biol Chem, 2022,298(4):101768
Li R, Xiao X, Yan Y, et al. GPRASP1 loss-of-function links to arteriovenous malformations by endothelial activating GPR4 signals. Brain, 2024,147(4):1571–1586
Singh LS, Berk M, Oates R, et al. Ovarian cancer G protein-coupled receptor 1, a new metastasis suppressor gene in prostate cancer. J Natl Cancer Inst, 2007,99(17):1313–1327
Xue S, Su Z, Liu D. Immunometabolism and immune response regulate macrophage function in atherosclerosis. Ageing Res Rev, 2023,90:101993
Ouyang S, Li Y, Wu X, et al. GPR4 signaling is essential for the promotion of acid-mediated angiogenic capacity of endothelial progenitor cells by activating STAT3/VEGFA pathway in patients with coronary artery disease. Stem Cell Res Ther, 2021,12(1):149
Li J, Guo B, Wang J, et al. Ovarian cancer G protein coupled receptor 1 suppresses cell migration of MCF7 breast cancer cells via a Galpha12/13-Rho-Rac1 pathway. J Mol Signal, 2013,8(1):6
Bell TJ, Nagel DJ, Woeller CF, et al. Ogerin mediated inhibition of TGF-β(1) induced myofibroblast differentiation is potentiated by acidic pH. PLoS One, 2022,17(7):e0271608
Sharma AL, Meitei PM, Machathoibi TC, et al. Ovarian cancer G protein-coupled receptor 1 inhibits A549 cells migration through casein kinase 2α intronless gene and neutral endopeptidase. BMC Cancer, 2022,22(1):143
Joost P, Methner A. Phylogenetic analysis of 277 human G-protein-coupled receptors as a tool for the prediction of orphan receptor ligands. Genome Biol, 2002,3(11):RESEARCH0063
Foord SM, Bonner TI, Neubig RR, et al. International Union of Pharmacology. XLVI. G protein-coupled receptor list. Pharmacol Rev, 2005,57(2):279–288
Fredriksson R, Lagerström MC, Lundin LG, et al. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol Pharmacol, 2003,63(6):1256–1272
Wheatley M, Wootten D, Conner MT, et al. Lifting the lid on GPCRs: the role of extracellular loops. Br J Pharmacol, 2012,165(6):1688–1703
Kim HR, Xu J, Maeda S, et al. Structural mechanism underlying primary and secondary coupling between GPCRs and the Gi/o family. Nat Commun, 2020,11(1):3160
Smith SO. Deconstructing the transmembrane core of class A G protein-coupled receptors. Trends Biochem Sci, 2021,46(12):1017–1029
Rowe JB, Kapolka NJ, Taghon GJ, et al. The evolution and mechanism of GPCR proton sensing. J Biol Chem, 2021,296:100167
Wang JQ, Kon J, Mogi C, et al. TDAG8 is a proton-sensing and psychosine-sensitive G-protein-coupled receptor. J Biol Chem, 2004,279(44):45626–45633
Radu CG, Nijagal A, McLaughlin J, et al. Differential proton sensitivity of related G protein-coupled receptors T cell death-associated gene 8 and G2A expressed in immune cells. Proc Natl Acad Sci USA, 2005,102(5):1632–1637
Choi JW, Lee SY, Choi Y. Identification of a putative G proteincoupled receptor induced during activation-induced apoptosis of T cells. Cell Immunol, 1996,168(1):78–84
Fagerberg L, Hallström BM, Oksvold P, et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol Cell Proteomics, 2014,13(2):397–406
Simon MI, Strathmann MP, Gautam N. Diversity of G proteins in signal transduction. Science, 1991,252(5007):802–808
An S, Tsai C, Goetzl EJ. Cloning, sequencing and tissue distribution of two related G protein-coupled receptor candidates expressed prominently in human lung tissue. FEBS Lett, 1995,375(1–2):121–124
Li R, Guan Z, Bi S, et al. The proton-activated G protein-coupled receptor GPR4 regulates the development of osteoarthritis via modulating CXCL12/CXCR7 signaling. Cell Death Dis, 2022,13(2):152
Liu JP, Nakakura T, Tomura H, et al. Each one of certain histidine residues in G-protein-coupled receptor GPR4 is critical for extracellular proton-induced stimulation of multiple G-protein-signaling pathways. Pharmacol Res, 2010,61(6):499–505
Tobo M, Tomura H, Mogi C, et al. Previously postulated “ligand-independent” signaling of GPR4 is mediated through proton-sensing mechanisms. Cell Signal, 2007,19(8):1745–1753
Chen A, Dong L, Leffler NR, et al. Activation of GPR4 by acidosis increases endothelial cell adhesion through the cAMP/Epac pathway. PLoS One, 2011,6(11):e27586
Dong L, Li Z, Leffler NR, et al. Acidosis activation of the proton-sensing GPR4 receptor stimulates vascular endothelial cell inflammatory responses revealed by transcriptome analysis. PLoS One, 2013,8(4):e61991
Okito A, Nakahama KI, Akiyama M, et al. Involvement of the G-protein-coupled receptor 4 in RANKL expression by osteoblasts in an acidic environment. Biochem Biophys Res Commun, 2015,458(2):435–440
Krewson EA, Sanderlin EJ, Marie MA, et al. The Proton-Sensing GPR4 Receptor Regulates Paracellular Gap Formation and Permeability of Vascular Endothelial Cells. iScience, 2020,23(2):100848
Dong L, Krewson EA, Yang LV. Acidosis Activates Endoplasmic Reticulum Stress Pathways through GPR4 in Human Vascular Endothelial Cells. Int J Mol Sci, 2017,18(2):278
Wei WC, Bianchi F, Wang YK, et al. Coincidence Detection of Membrane Stretch and Extracellular pH by the Proton-Sensing Receptor OGR1 (GPR68). Curr Biol, 2018,28(23):3815–3823
de Vallière C, Cosin-Roger J, Simmen S, et al. Hypoxia Positively Regulates the Expression of pH-Sensing G-Protein-Coupled Receptor OGR1 (GPR68). Cell Mol Gastroenterol Hepatol, 2016,2(6):796–810
Pera T, Deshpande DA, Ippolito M, et al. Biased signaling of the proton-sensing receptor OGR1 by benzodiazepines. FASEB J, 2018,32(2):862–874
Saxena H, Deshpande DA, Tiegs BC, et al. The GPCR OGR1 (GPR68) mediates diverse signalling and contraction of airway smooth muscle in response to small reductions in extracellular pH. Br J Pharmacol, 2012,166(3):981–990
Maeyashiki C, Melhem H, Hering L, et al. Activation of pH-Sensing Receptor OGR1 (GPR68) Induces ER Stress Via the IRE1α/JNK Pathway in an Intestinal Epithelial Cell Model. Sci Rep, 2020,10(1):1438
Kotake M, Sato K, Mogi C, et al. Acidic pH increases cGMP accumulation through the OGR1/phospholipase C/Ca(2+)/neuronal NOS pathway in N1E-115 neuronal cells. Cell Signal, 2014,26(11):2326–2332
Jiang Z, Zhu J, Zhao C, et al. Corrigendum to (Shear Stress Regulates the SNAP23-mediated Endothelial secretion of VWF through the GPR68/PKA/vimentin Mechanotransduction Pathway) (BBRC, 2022,607:166–173]. Biochem Biophys Res Commun, 2022,609:195–196
Zhu C, Wang L, Zhu J, et al. OGR1 negatively regulates β-casein and triglyceride synthesis and cell proliferation via the PI3K/AKT/mTOR signaling pathway in goat mammary epithelial cells. Anim Biotechnol, 2021,32(5):627–636
Wang Y, de Vallière C, Imenez Silva PH, et al. The Proton-activated Receptor GPR4 Modulates Intestinal Inflammation. J Crohns Colitis, 2018,12(3):355–368
Williams CH, Neitzel LR, Cornell J, et al. GPR68-ATF4 signaling is a novel prosurvival pathway in glioblastoma activated by acidic extracellular microenvironment. Exp Hematol Oncol, 2024,13(1):13
Xie L, McKenzie CI, Qu X, et al. pH and Proton Sensor GPR65 Determine Susceptibility to Atopic Dermatitis. J Immunol, 2021,207(1):101–109
Xu X, Bu B, Tian H, et al. MicroRNAs combined with the TLR4/TDAG8 mRNAs and proinflammatory cytokines are biomarkers for the rapid diagnosis of sepsis. Mol Med Rep, 2022,26(5):334
Mercier V, Boucher G, Devost D, et al. IBD-associated G protein-coupled receptor 65 variant compromises signalling and impairs key functions involved in inflammation. Cell Signal, 2022,93:110294
Zhang K, Zhang MX, Meng XX, et al. Targeting GPR65 alleviates hepatic inflammation and fibrosis by suppressing the JNK and NF-κB pathways. Mil Med Res, 2023,10(1):56
Lassen KG, McKenzie CI, Mari M, et al. Genetic Coding Variant in GPR65 Alters Lysosomal pH and Links Lysosomal Dysfunction with Colitis Risk. Immunity, 2016,44(6):1392–1405
Huang XP, Karpiak J, Kroeze WK, et al. Allosteric ligands for the pharmacologically dark receptors GPR68 and GPR65. Nature, 2015,527(7579):477–483
Lin R, Wu W, Chen H, et al. GPR65 promotes intestinal mucosal Th1 and Th17 cell differentiation and gut inflammation through downregulating NUAK2. Clin Transl Med, 2022,12(3):e771
Jin Y, Sato K, Tobo A, et al. Inhibition of interleukin-1β production by extracellular acidification through the TDAG8/cAMP pathway in mouse microglia. J Neurochem, 2014,129(4):683–695
Ma XD, Hang LH, Shao DH, et al. TDAG8 activation attenuates cerebral ischaemia-reperfusion injury via Akt signalling in rats. Exp Neurol, 2017,293:115–123
Sayama K, Yuki K, Sugata K, et al. Carbon dioxide inhibits UVB-induced inflammatory response by activating the proton-sensing receptor, GPR65, in human keratinocytes. Sci Rep, 2021,11(1):379
Mao J, Feng Y, Zheng Y, et al. GPR65 inhibits human trophoblast cell adhesion through upregulation of MYLK and downregulation of fibronectin via cAMP-ERK signaling in a low pH environment. Cell Commun Signal, 2023,21(1):238
Onozawa Y, Komai T, Oda T. Activation of T cell death-associated gene 8 attenuates inflammation by negatively regulating the function of inflammatory cells. Eur J Pharmacol, 2011,654(3):315–319
Kottyan LC, Collier AR, Cao KH, et al. Eosinophil viability is increased by acidic pH in a cAMP- and GPR65-dependent manner. Blood, 2009,114(13):2774–2782
Weng Z, Fluckiger AC, Nisitani S, et al. A DNA damage and stress inducible G protein-coupled receptor blocks cells in G2/M. Proc Natl Acad Sci USA, 1998,95(21):12334–12339
Kabarowski JH, Feramisco JD, Le LQ, et al. Direct genetic demonstration of G alpha 13 coupling to the orphan G protein-coupled receptor G2A leading to RhoA-dependent actin rearrangement. Proc Natl Acad Sci USA, 2000,97(22):12109–12114
Kabarowski JH, Zhu K, Le LQ, et al. Lysophosphatidylcholine as a ligand for the immunoregulatory receptor G2A. Science, 2001,293(5530):702–705
Hasegawa H, Lei J, Matsumoto T, et al. Lysophosphatidylcholine enhances the suppressive function of human naturally occurring regulatory T cells through TGF-β production. Biochem Biophys Res Commun, 2011,415(3):526–531
Mogi C, Tobo M, Tomura H, et al. Involvement of proton-sensing TDAG8 in extracellular acidification-induced inhibition of proinflammatory cytokine production in peritoneal macrophages. J Immunol, 2009,182(5):3243–3251
Chong YH, Shin SA, Lee HJ, et al. Molecular mechanisms underlying cyclic AMP inhibition of macrophage dependent TNF-alpha production and neurotoxicity in response to amyloidogenic C-terminal fragment of Alzheimer’s amyloid precursor protein. J Neuroimmunol, 2002,133(1–2):160–174
Tcymbarevich I, Richards SM, Russo G, et al. Lack of the pH-sensing Receptor TDAG8 [GPR65] in Macrophages Plays a Detrimental Role in Murine Models of Inflammatory Bowel Disease. J Crohns Colitis, 2019,13(2):245–258
de Vallière C, Cosin-Roger J, Simmen S, et al. Hypoxia Positively Regulates the Expression of pH-Sensing G-Protein-Coupled Receptor OGR1 (GPR68). Cell Mol Gastroenterol Hepatol, 2016,2(6):796–810
Ichimonji I, Tomura H, Mogi C, et al. Extracellular acidification stimulates IL-6 production and Ca(2+) mobilization through proton-sensing OGR1 receptors in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol, 2010,299(4):L567–L577
Yassini N, Sprenger J, Pastor Arroyo EM, et al. Ovarian cancer G protein-coupled receptor 1 deficiency exacerbates crystal deposition and kidney injury in oxalate nephropathy in female mice. Clin Sci (Lond), 2023,137(14):1013–1025
Yan L, Singh LS, Zhang L, et al. Role of OGR1 in myeloid-derived cells in prostate cancer. Oncogene, 2014,33(2):157–164
Umemura N, Saio M, Suwa T, et al. Tumor-infiltrating myeloid-derived suppressor cells are pleiotropic-inflamed monocytes/macrophages that bear M1- and M2-type characteristics. J Leukoc Biol, 2008,83(5):1136–1144
Bolick DT, Skaflen MD, Johnson LE, et al. G2A deficiency in mice promotes macrophage activation and atherosclerosis. Circ Res, 2009,104(3):318–327
Kern K, Schäfer SMG, Cohnen J, et al. The G2A Receptor Controls Polarization of Macrophage by Determining Their Localization Within the Inflamed Tissue. Front Immunol, 2018,9:2261
Zhu X, Mose E, Hogan SP, et al. Differential eosinophil and mast cell regulation: mast cell viability and accumulation in inflammatory tissue are independent of proton-sensing receptor GPR65. Am J Physiol Gastrointest Liver Physiol, 2014,306(11):G974–G982
Liu S, Chirkov YY, Horowitz JD. Neutrophil-Initiated Myocardial Inflammation and Its Modulation by B-Type Natriuretic Peptide: A Potential Therapeutic Target. Int J Mol Sci, 2018;20(1):129
Murata N, Mogi C, Tobo M, et al. Inhibition of superoxide anion production by extracellular acidification in neutrophils. Cell Immunol, 2009,259(1):21–26
Tsurumaki H, Mogi C, Aoki-Saito H, et al. Protective Role of Proton-Sensing TDAG8 in Lipopolysaccharide-Induced Acute Lung Injury. Int J Mol Sci, 2015,16(12):28931–28942
Khan SY, McLaughlin NJD, Kelher MR, et al. Lysophosphatidylcholines activate G2A inducing G(αi)−1-/G(αq/)11- Ca2(+) flux, G(βγ)-Hck activation and clathrin/β-arrestin-1/GRK6 recruitment in PMNs. Biochem J, 2010,432(1):35–45
Frasch SC, Fernandez-Boyanapalli RF, Berry KAZ, et al. Neutrophils regulate tissue Neutrophilia in inflammation via the oxidant-modified lipid lysophosphatidylserine. J Biol Chem, 2013,288(7):4583–4593
Tosa N, Murakami M, Jia WY, et al. Critical function of T cell death-associated gene 8 in glucocorticoid-induced thymocyte apoptosis. Int Immunol, 2003,15(6):741–749
Chen X, Jaiswal A, Costliow Z, et al. pH sensing controls tissue inflammation by modulating cellular metabolism and endo-lysosomal function of immune cells. Nat Immunol, 2022,23(7):1063–1075
Sanderlin EJ, Leffler NR, Lertpiriyapong K, et al. GPR4 deficiency alleviates intestinal inflammation in a mouse model of acute experimental colitis. Biochim Biophys Acta Mol Basis Dis, 2017,1863(2):569–584
Aoki H, Mogi C, Hisada T, et al. Proton-sensing ovarian cancer G protein-coupled receptor 1 on dendritic cells is required for airway responses in a murine asthma model. PLoS One, 2013,8(11):e79985
Aoki H, Mogi C, Okajima F. Ionotropic and metabotropic proton-sensing receptors involved in airway inflammation in allergic asthma. Mediators Inflamm, 2014,2014:712962
D’Souza CA, Zhao FL, Li X, et al. OGR1/GPR68 Modulates the Severity of Experimental Autoimmune Encephalomyelitis and Regulates Nitric Oxide Production by Macrophages. PLoS One, 2016,11(2):e0148439
Dai SP, Hsieh WS, Chen CH, et al. TDAG8 deficiency reduces satellite glial number and pro-inflammatory macrophage number to relieve rheumatoid arthritis disease severity and chronic pain. J Neuroinflammation, 2020,17(1):170
Gaublomme JT, Yosef N, Lee Y, et al. Single-Cell Genomics Unveils Critical Regulators of Th17 Cell Pathogenicity. Cell, 2015,163(6):1400–1412
Osmers I, Smith SS, Parks BW, et al. Deletion of the G2A receptor fails to attenuate experimental autoimmune encephalomyelitis. J Neuroimmunol, 2009,207(1–2):18–23
Li J, Chen K, Zhao Z. The protective effects of NE 52-QQ57 against interleukin-33-induced inflammatory response in activated synovial mast cells. J Biochem Mol Toxicol, 2022,36(8):e23116
Velcicky J, Miltz W, Oberhauser B, et al. Development of Selective, Orally Active GPR4 Antagonists with Modulatory Effects on Nociception, Inflammation, and Angiogenesis. J Med Chem 2017,60(9):3672–3683
Onozawa Y, Fujita Y, Kuwabara H, et al. Activation of T cell death-associated gene 8 regulates the cytokine production of T cells and macrophages in vitro. Eur J Pharmacol, 2012,683(1–3):325–331
Sanderlin EJ, Marie M, Velcicky J, et al. Pharmacological inhibition of GPR4 remediates intestinal inflammation in a mouse colitis model. Eur J Pharmacol, 2019,852:218–230
Stalewski J, Shih AY, Papazyan R, et al. pH Dependence of a GPR4 Selective Antagonist Hampers Its Therapeutic Potential. J Pharmacol Exp Ther, 2023,386(1):35–44
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest.
Additional information
This study was supported by the National Nature Science Foundation of China (No. 81873694), the Key Research and Development Program of Hubei Province (No. 2022BCA005), and Knowledge Innovation Program of Wuhan Basic Research (No. 2022020801010446).
Rights and permissions
About this article
Cite this article
Li, Ms., Wang, Xh. & Wang, H. Immunomodulation of Proton-activated G Protein-coupled Receptors in Inflammation. CURR MED SCI (2024). https://doi.org/10.1007/s11596-024-2872-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11596-024-2872-4