Abstract
Objective
Post-stroke cognitive impairment (PSCI) develops in approximately one-third of stroke survivors and is associated with ingravescence. Nonetheless, the biochemical mechanisms underlying PSCI remain unclear. The study aimed to establish an ischemic mouse model by means of transient unilateral middle cerebral artery occlusions (MCAOs) and to explore the biochemical mechanisms of p25/cyclin-dependent kinase 5 (CDK5)-mediated tau hyperphosphorylation on the PSCI behavior.
Methods
Cognitive behavior was investigated, followed by the detection of tau hyperphosphorylation, mobilization, activation of kinases and/or inhibition of phosphatases in the lateral and contralateral cerebrum of mice following ischemia in MACO mice. Finally, we treated HEK293/tau cells with oxygen-glucose deprivation (OGD) and a CDK5 inhibitor (Roscovitine) or a GSK3β inhibitor (LiCl) to the roles of CDK5 and GSK3β in mediating ischemia-reperfusion-induced tau phosphorylation.
Results
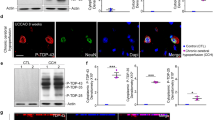
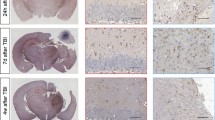
Ischemia induced cognitive impairments within 2 months, as well as causing tau hyperphosphorylation and its localization to neuronal somata in both ipsilateral and contralateral cerebra. Furthermore, p25 that promotes CDK5 hyperactivation had significantly higher expression in the mice with MCAO than in the shamoperation (control) group, while the expression levels of protein phosphatase 2 (PP2A) and the phosphorylation level at Tyr307 were comparable between the two groups. In addition, the CDK5 inhibitor rescued tau from hyperphosphorylation induced by OGD.
Conclusion
These findings demonstrate that upregulation of CDK5 mediates tau hyperphosphorylation and localization in both ipsilateral and contralateral cerebra, contributing to the pathogenesis of PSCI.
Similar content being viewed by others
References
Tatemichi TK, Paik M, Bagiella E, et al. Risk of dementia after stroke in a hospitalized cohort: results of a longitudinal study. Neurology, 1994,44(10):1885–1891
Loeb C, Gandolfo C, Croce R, et al. Dementia associated with lacunar infarction. Stroke, 1992,23(9):1225–1229
Allan LM, Rowan EN, Firbank MJ, et al. Long term incidence of dementia, predictors of mortality and pathological diagnosis in older stroke survivors. Brain, 2011,134(Pt 12):3716–3727
Tatemichi TK, Desmond DW, Mayeux R, et al. Dementia after stroke: baseline frequency, risks, and clinical features in a hospitalized cohort. Neurology, 1992,42(6):1185–1193
Fride Y, Adamit T, Maeir A, et al. What are the correlates of cognition and participation to return to work after first ever mild stroke?. Top Stroke Rehabil, 2015,22(5):317–325
Mijajlovic MD, Pavlovic A, Brainin M, et al. Post-stroke dementia - a comprehensive review. BMC Med, 2017,15(1):11
Yang C, Hawkins KE, Dore S, et al. Neuroinflammatory mechanisms of blood-brain barrier damage in ischemic stroke. Am J Physiol Cell Physiol, 2019,316(2):C135–C153
Doyle KP, Simon RP, Stenzel-Poore MP. Mechanisms of ischemic brain damage. Neuropharmacology, 2008,55(3):310–318
Wu M, Zhang M, Yin X, et al. The role of pathological tau in synaptic dysfunction in Alzheimer’s diseases. Transl Neurodegener, 2021,10(1):45
Wang JZ, Gao X, Wang ZH. The physiology and pathology of microtubule-associated protein tau. Essays Biochem, 2014, 56: 111–123
Alonso AC, Zaidi T, Grundke-Iqbal I, et al. Role of abnormally phosphorylated tau in the breakdown of microtubules in Alzheimer disease. Proc Natl Acad Sci USA, 1994,91(12):5562–5566
Sen T, Saha P, Jiang T, et al. Sulfhydration of AKT triggers Tau-phosphorylation by activating glycogen synthase kinase 3beta in Alzheimer’s disease. Proc Natl Acad Sci USA, 2020,117(8):4418–4427
Hoover BR, Reed MN, Su J, et al. Tau Mislocalization to Dendritic Spines Mediates Synaptic Dysfunction Independently of Neurodegeneration. Neuron, 2010,68(6):1067–1081
Pao PC, Tsai LH. Three decades of Cdk5. J Biomed Sci, 2021,28(1):79
Ao C, Li C, Chen J, et al. The role of Cdk5 in neurological disorders. Front Cell Neurosci, 2022, 16: 951202
Patrick GN, Zukerberg L, Nikolic M, et al. Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature, 1999,402(6762):615–622
Lee M S, Kwon Y T, Li M, et al. Neurotoxicity induces cleavage of p35 to p25 by calpain. Nature, 2000,405(6784):360–364
Dhavan R, Tsai LH. A decade of CDK5. Nat Rev Mol Cell Biol, 2001,2(10):749–759
Cheung ZH, Fu AKY, Ip NY. Synaptic roles of Cdk5: Implications in higher cognitive functions and neurodegenerative diseases. Neuron, 2006,50(1):13–18
Arioka M, Tsukamoto M, Ishiguro K, et al. Tau protein kinase II is involved in the regulation of the normal phosphorylation state of tau protein. J Neurochem, 1993,60(2):461–468
Kimura T, Ishiguro K, Hisanaga S. Physiological and pathological phosphorylation of tau by Cdk5. Front Mol Neurosci, 2014, 7: 65
Shukla V, Skuntz S, Pant HC. Deregulated Cdk5 Activity Is Involved in Inducing Alzheimer’s Disease. Arch Med Res, 2012,43(8):655–662
Tuo QZ, Lei P, Jackman KA, et al. Tau-mediated iron export prevents ferroptotic damage after ischemic stroke. Mol Psychiatry, 2017,22(11):1520–1530
Li DJ, Li YH, Yuan HB, et al. The novel exercise-induced hormone irisin protects against neuronal injury via activation of the Akt and ERK1/2 signaling pathways and contributes to the neuroprotection of physical exercise in cerebral ischemia. Metabolism, 2017, 68: 31–42
Kubota T, Kirino Y. Age-dependent impairment of memory and neurofibrillary tangle formation and clearance in a mouse model of tauopathy. Brain Res, 2021, 1765: 147496
Ramsden M, Kotilinek L, Forster C, et al. Age-dependent neurofibrillary tangle formation, neuron loss, and memory impairment in a mouse model of human tauopathy (P301L). J Neurosci, 2005,25(46):10637–10647
Paonessa F, Evans LD, Solanki R, et al. Microtubules Deform the Nuclear Membrane and Disrupt Nucleocytoplasmic Transport in Tau-Mediated Frontotemporal Dementia. Cell Reports, 2019,26(3):582–593
Shin MK, Vazquez-Rosa E, Koh Y, et al. Reducing acetylated tau is neuroprotective in brain injury. Cell, 2021,184(10):2715–2732
Wang JZ, Grundke-Iqbal I, Iqbal K. Kinases and phosphatases and tau sites involved in Alzheimer neurofibrillary degeneration. Eur J Neurosci, 2007,25(1):59–68
Hanger DP, Noble W. Functional implications of glycogen synthase kinase-3-mediated tau phosphorylation. Int J Alzheimers Dis, 2011, 2011: 352805
Gong CX, Shaikh S, Wang JZ, et al. Phosphatase activity toward abnormally phosphorylated tau: decrease in Alzheimer disease brain. J Neurochem, 1995,65(2):732–738
Sandal P, Jong CJ, Merrill RA, et al. Protein phosphatase 2A-structure, function and role in neurodevelopmental disorders. J Cell Sci, 2021,134(13):248187
Seshadri S, Wolf PA. Lifetime risk of stroke and dementia: current concepts, and estimates from the Framingham Study. Lancet Neurol, 2007,6(12):1106–1114
Kalmijn S, Launer LJ, Lindemans J, et al. Total homocysteine and cognitive decline in a community-based sample of elderly subjects: the Rotterdam Study. Am J Epidemiol, 1999,150(3):283–289
Rusanen M, Kivipelto M, Quesenberry CP, et al. Heavy smoking in midlife and long-term risk of Alzheimer disease and vascular dementia. Arch Intern Med, 2011,171(4):333–339
Tamaoka A, Kalaria RN, Lieberburg I, et al. Identification of a stable fragment of the Alzheimer amyloid precursor containing the beta-protein in brain microvessels. Proc Natl Acad Sci USA, 1992,89(4):1345–1349
Fujii H, Takahashi T, Mukai T, et al. Modifications of tau protein after cerebral ischemia and reperfusion in rats are similar to those occurring in Alzheimer’s disease - Hyperphosphorylation and cleavage of 4- and 3-repeat tau. J Cereb Blood Flow Metab, 2017,37(7):2441–2457
Martin L, Latypova X, Wilson CM, et al. Tau protein kinases: involvement in Alzheimer’s disease. Ageing Res Rev, 2013,12(1):289–309
Hanger DP, Anderton BH, Noble W. Tau phosphorylation: the therapeutic challenge for neurodegenerative disease. Trends Mol Med, 2009,15(3):112–119
Liu YQ, Kong C, Gong L, et al. The Association of Post-Stroke Cognitive Impairment and Gut Microbiota and its Corresponding Metabolites. J Alzheimers Dis, 2020,73(4):1455–1466
Cai HY, Zhao ZY, Ni LH, et al. Structural and Functional Deficits in Patients with Poststroke Dementia: A Multimodal MRI Study. Neural Plast, 2021:3536234
Hilkens NA, Klijn CJM, Richard E. Blood pressure, blood pressure variability and the risk of poststroke dementia. J Hypertens, 2021,39(9):1859–1864
Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol, 2009,8(11):1006–1018
Levine DA, Galecki AT, Langa KM, et al. Risk Factors for Poststroke Cognitive Decline: The REGARDS Study (Reasons for Geographic and Racial Differences in Stroke). Stroke, 2018,49(4):987–994
Levine DA, Galecki AT, Langa KM, et al. Trajectory of Cognitive Decline After Incident Stroke. JAMA, 2015,314(1):41–51
Pollock A, St George B, Fenton M, et al. Top ten research priorities relating to life after stroke. Lancet Neurol, 2012,11(3):209–209
Martin L, Latypova X, Wilson CM, et al. Tau protein phosphatases in Alzheimer’s disease: The leading role of PP2A. Ageing Res Rev, 2013,12(1):39–49
El KN, Gratuze M, Papon MA, et al. Insulin dysfunction and Tau pathology. Front Cell Neurosci, 2014, 8: 22
Planel E, Tatebayashi Y, Miyasaka T, et al. Insulin dysfunction induces in vivo tau hyperphosphorylation through distinct mechanisms. J Neurosci, 2007,27(50):13635–13648
Zhang Y, Huang NQ, Yan F, et al. Diabetes mellitus and Alzheimer’s disease: GSK-3 beta as a potential link. Behav Brain Res, 2018, 339: 57–65
Liu Y, Liu F, Grundke-Iqbal I, et al. Deficient brain insulin signalling pathway in Alzheimer’s disease and diabetes. J Pathol, 2011,225(1):54–62
Goncalves RA, Wijesekara N, Fraser PE, et al. The Link Between Tau and Insulin Signaling: Implications for Alzheimer’s Disease and Other Tauopathies. Front Cell Neurosci, 2019, 13: 17
Yang LY, Wang HY, Liu LJ, et al. The Role of Insulin/IGF-1/PI3K/Akt/GSK3 beta Signaling in Parkinson’s Disease Dementia. Front Neurosci, 2018, 12: 73
Gabbouj S, Ryhanen S, Marttinen M, et al. Altered Insulin Signaling in Alzheimer’s Disease Brain - Special Emphasis on PI3K-Akt Pathway. Front Neurosci, 2019, 13: 629
Sun KH, de Pablo Y, Vincent F, et al. Novel genetic tools reveal Cdk5’s major role in Golgi fragmentation in Alzheimer’s disease. Mol Biol Cell, 2008,19(7):3052–3069
Wei FY, Tomizawa K. Cyclin-dependent kinase 5 (Cdk5): a potential therapeutic target for the treatment of neurodegenerative diseases and diabetes mellitus. Mini Rev Med Chem, 2007,7(10):1070–1074
Meyer DA, Torres-Altoro MI, Tan ZJ, et al. Ischemic Stroke Injury Is Mediated by Aberrant Cdk5. J Neurosci, 2014,34(24):8259–8267
Smith PD, Crocker SJ, Jackson-Lewis V, et al. Cyclin-dependent kinase 5 is a mediator of dopaminergic neuron loss in a mouse model of Parkinson’s disease. Proc Natl Acad Sci USA, 2003,100(23):13650–13655
Gutierrez-Vargas JA, Moreno H, Cardona-Gomez GP. Targeting CDK5 post-stroke provides long-term neuroprotection and rescues synaptic plasticity. J Cereb Blood Flow Metab, 2017,37(6):2208–2223
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare no conflict of interest.
Additional information
This research was funded by grants from the National Natural Science Foundation of China (No. 31800851), Natural Science Foundation of Hubei Province (No. 2022CFB456) and The Research Fund of Jianghan University (No. 08210011).
Supplementary data
Rights and permissions
About this article
Cite this article
Yu, J., Zhao, Y., Gong, Xk. et al. P25/CDK5-mediated Tau Hyperphosphorylation in Both Ipsilateral and Contralateral Cerebra Contributes to Cognitive Deficits in Post-stroke Mice. CURR MED SCI 43, 1084–1095 (2023). https://doi.org/10.1007/s11596-023-2792-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-023-2792-8