Abstract
Objective
This study aimed to explore the value of M701, targeting epithelial cell adhesion molecule (EpCAM) and CD3, in the immunotherapy of ovarian cancer ascites by the in vitro assay.
Methods
The expression of EpCAM in ovarian cancer tissues was analyzed by databases. The EpCAM expression and immune cell infiltration in different foci of ovarian cancer were detected by 8-channel flow cytometry. The toxic effect of M701 on OVCAR3 was tested using the in vitro cytotoxicity assay. The 3D cell culture and drug intervention experiments were performed to evaluate the therapeutic effect of M701 in ovarian cancer specimens. Flow cytometry was used to examine the effect of M701 on the binding of immune cells to tumor cells and the activation capacity of T cells.
Results
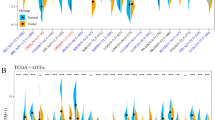
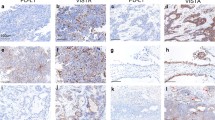
The results of the bioinformatic analysis showed that the expression of EpCAM in ovarian cancer tissue was significantly higher than that in normal ovarian tissue. The 8-channel flow cytometry of clinical samples showed that the EpCAM expression and lymphocyte infiltration were significantly heterogeneous among ovarian cancer patients and lesions at different sites. The in vitro experiment results showed that M701 had a significant killing effect on OVCAR3 cells. M701 also obviously killed primary tumor cells derived from some patients with ovarian cancer ascites. M701 could mediate the binding of CD3+ T cells to EpCAM+ tumor cells and induce T cell activation in a dose-dependent manner.
Conclusion
M701 showed significant inhibitory activity on tumor cells derived from ovarian cancer ascites, which had a promising application in immunotherapy for patients with ovarian cancer ascites.
Similar content being viewed by others
References
Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin, 2021,71(3):209–249
Reid BM, Permuth JB, Sellers TA. Epidemiology of ovarian cancer: a review. Cancer Biol Med, 2017,14(1):9–32
Ferriss JS, Java JJ, Bookman MA, et al. Ascites predicts treatment benefit of bevacizumab in front-line therapy of advanced epithelial ovarian, fallopian tube and peritoneal cancers: An NRG Oncology/GOG study. Gynecol Oncol, 2015,139(1):17–22
Baert T, Ferrero A, Sehouli J, et al. The systemic treatment of recurrent ovarian cancer revisited. Ann Oncol, 2021,32(6):710–725
den Ouden JE, Zaman GJR, Dylus J, et al. Chemotherapy sensitivity testing on ovarian cancer cells isolated from malignant ascites. Oncotarget, 2020,11(49):4570–4581
Kipps E, Tan DSP, Kaye SB. Meeting the challenge of ascites in ovarian cancer: new avenues for therapy and research. Nat Rev Cancer, 2013,13(4):273–282
Gallego A, Mendiola M, Hernando B, et al. Prognostic markers of inflammation in endometrioid and clear cell ovarian cancer. Int J Gynecol Cancer, 2022,32(8):1009–1016
Jlassi A, Manai M, Morjen M, et al. VISTA+/CD8+status correlates with favorable prognosis in Epithelial ovarian cancer. PLoS One, 2023,18(3):e0278849–e
Ukita M, Hamanishi J, Yoshitomi H, et al. CXCL13-producing CD4+T cells accumulate in the early phase of tertiary lymphoid structures in ovarian cancer. JCI Insight, 2022,7(12):e157215
Amblard E, Soumelis V. Context-Dependent Effects Explain Divergent Prognostic Roles of Tregs in Cancer. Cancers, 2022,14(12):2991
Bhandaru M, Rotte A. Monoclonal Antibodies for the Treatment of Melanoma: Present and Future Strategies. Methods Mol Biol, 2019,1904:83–108
Wudhikarn K, King AC, Geyer MB, et al. Outcomes of relapsed B-cell acute lymphoblastic leukemia after sequential treatment with blinatumomab and inotuzumab. Blood Adv, 2022,6(5):1432–1443
Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science, 2018,359(6382):1350–1355
Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science, 2015,348(6230):62–68
Ni L, Lu J. Interferon gamma in cancer immunotherapy. Cancer Med, 2018,7(9):4509–4516
Morand S, Devanaboyina M, Staats H, et al. Ovarian Cancer Immunotherapy and Personalized Medicine. Int J Mol Sci, 2021,22(12):6532
Mashhadi SMY, Kazemimanesh M, Arashkia A, et al. Shedding light on the EpCAM: An overview. J Cell Physiol, 2019,234(8):12569–12580
Patriarca C, Macchi RM, Marschner AK, et al. Epithelial cell adhesion molecule expression (CD326) in cancer: A short review. Cancer Treat Rev, 2012,38(1):68–75
Herreros-Pomares A, Aguilar-Gallardo C, Calabuig-Farinas S, et al. EpCAM duality becomes this molecule in a new Dr. Jekyll and Mr. Hyde tale. Crit Rev Oncol Hematol, 2018,126:52–63
Seeber A, Untergasser G, Spizzo G, et al. Predominant expression of truncated EpCAM is associated with a more aggressive phenotype and predicts poor overall survival in colorectal cancer. Int J Cancer, 2016,139(3):657–663
Massoner P, Thomm T, Mack B, et al. EpCAM is overexpressed in local and metastatic prostate cancer, suppressed by chemotherapy and modulated by MET-associated miRNA-200c/205. Br J Cancer, 2014,111(5):955–964
Spizzo G, Went P, Dirnhofer S, et al. Overexpression of epithelial cell adhesion molecule (Ep-CAM) is an independent prognostic marker for reduced survival of patients with epithelial ovarian cancer. Gynecol Oncol, 2006,103(2): 483–488
Brunner A, Prelog M, Verdorfer I, et al. EpCAM is predominantly expressed in high grade and advanced stage urothelial carcinoma of the bladder. J Clin Pathol, 2008,61(3):307–310
Fong D, Moser P, Kasal A, et al. Loss of membranous expression of the intracellular domain of EpCAM is a frequent event and predicts poor survival in patients with pancreatic cancer. Histopathology, 2014,64(5):683–692
Lee SJ, Chung KY, Kwon JE, et al. Expression of EpCAM in adenoid cystic carcinoma. Pathology, 2018,50(7):737–741
Gaghana LO, Miskad U, Cangara MH, et al. The Relationship Between Expression of EpCAM Cancer Stem Cell Marker with Histopathological Grading, Lymphovascular Invasion, and Metastases in Colorectal Adenocarcinoma. Asian Pac J Cancer Prev, 2023,24(3):929–934
Yang Y, Fei F, Song Y, et al. Polymorphisms of EpCAM gene and prognosis for non-small-cell lung cancer in Han Chinese. Cancer Sci, 2014,105(1):89–96
Liao Y, Wu M, Jia Y, et al. EpCAM as a Novel Biomarker for Survivals in Prostate Cancer Patients. Front Cell Dev Biol, 2022,10:843604
Spizzo G, Fong D, Wurm M, et al. EpCAM expression in primary tumour tissues and metastases: an immunohistochemical analysis. J Clin Pathol, 2011, 64(5):415–420
van der Fels CAM, Rosati S, de Jong IJ. EpCAM Expression in Lymph Node Metastases of Urothelial Cell Carcinoma of the Bladder: A Pilot Study. Int J Mol Sci, 2017,18(8):1802
Liu X, Li J, Cadilha BL, et al. Epithelial-type systemic breast carcinoma cells with a restricted mesenchymal transition are a major source of metastasis. Sci Adv, 2019,5(6):eaav4275
Bellone S, Siegel ER, Cocco E, et al. Overexpression of Epithelial Cell Adhesion Molecule in Primary, Metastatic, and Recurrent/Chemotherapy-Resistant Epithelial Ovarian Cancer Implications for Epithelial Cell Adhesion Molecule-Specific Immunotherapy. Int J Gynecol Cancer, 2009,19(5):860–866
Fu J, Shang Y, Qian Z, et al. Chimeric antigen receptor-T (CAR-T) cells targeting epithelial cell adhesion molecule (EpCAM) can inhibit tumor growth in ovarian cancer mouse model. J Vet Med Sci, 2021,83(2):241–247
van den Brand D, van Lith SAM, de Jong JM, et al. EpCAM-Binding DARPins for Targeted Photodynamic Therapy of Ovarian Cancer. Cancers, 2020, 12(7):1762
Lee S, Ahn HJ. Anti-EpCAM-conjugated adeno-associated virus serotype 2 for systemic delivery of EGFR shRNA: Its retargeting and antitumor effects on OVCAR3 ovarian cancer in vivo. Acta Biomater, 2019,91:258–269
Zheng J, Zhao S, Yu X, et al. Simultaneous targeting of CD44 and EpCAM with a bispecific aptamer effectively inhibits intraperitoneal ovarian cancer growth. Theranostics, 2017,7(5):1373–1388
Wang L, Qiao Y, Zong H, et al. IgG-like Bispecific Antibody CD3xEpCAM Generated by Split Intein Against Colorectal Cancer. Front Pharmacol, 2022,13: 803059
Huehls AM, Coupet TA, Sentman CL. Bispecific T-cell engagers for cancer immunotherapy. Immunol Cell Biol, 2015,93(3):290–296
Seimetz D. Novel Monoclonal Antibodies for Cancer Treatment: The Trifunctional Antibody Catumaxomab (Removab (R)). J Cancer, 2011,2:309–316
Frampton JE. Catumaxomab in Malignant Ascites. Drugs, 2012,72(10): 1399–1410
Heiss MM, Murawa P, Koralewski P, et al. The trifunctional antibody catumaxomab for the treatment of malignant ascites due to epithelial cancer: results of a prospective randomized phase II/III trial. Int J Cancer, 2010,127(9): 2209–2221
English DP, Bellone S, Schwab CL, et al. Solitomab, an Epithelial Cell Adhesion Molecule/CD3 Bispecific Antibody (BiTE), Is Highly Active Against Primary Chemotherapy-Resistant Ovarian Cancer Cell Lines In Vitro and Fresh Tumor Cells Ex Vivo. Cancer, 2015,121(3):403–412
Song L, Xue J, Zhang J, et al. Mechanistic prediction of first-in-human dose for bispecific CD3/EpCAM T-cell engager antibody M701, using an integrated PK/PD modeling method. Eur J Pharm Sci, 2021,158:105584
Zhang CM, Yu LY, Lv JF, et al. Effects of immuno-related gene polymorphisms on a bispecific antibody targeting colorectal cancer cell. Per Med, 2018,15(3):167–179
Giornelli GH. Management of relapsed ovarian cancer: a review. Springerplus, 2016,5(1):1197
Keyvani V, Farshchian M, Esmaeili SA, et al. Ovarian cancer stem cells and targeted therapy. J Ovarian Res, 2019,12(1):120
Ning F, Cole CB, Annunziata CM. Driving Immune Responses in the Ovarian Tumor Microenvironment. Front Oncol, 2021,10:604084
Melichar B, Touskova M, Tosner J, et al. The phenotype of ascitic fluid lymphocytes in patients with ovarian carcinoma and other primaries. Onkologie, 2001,24(2):156–160
Fagotto F, Aslemarz A. EpCAM cellular functions in adhesion and migration, and potential impact on invasion: A critical review. Biochim Biophys Acta Rev Cancer, 2020,1874(2):188436
Landskron J, Helland O, Torgersen KM, et al. Activated regulatory and memory T-cells accumulate in malignant ascites from ovarian carcinoma patients. Cancer Immunol Immunother, 2015,64(3):337–347
Sato E, Olson SH, Ahn J, et al. Intraepithelial CD8(+) tumor-infiltrating lymphocytes and a high CD8(+)/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. PNAS, 2005,102(51):18538–18543
Zhang L, Conejo-Garcia JR, Katsaros D, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med, 2003,348(3): 203–213
Linke R, Klein A, Seimetz D. Catumaxomab Clinical development and future directions. Mabs, 2010,2(2):129–136
Hong R, Zhou Y, Tian X, et al. Selective inhibition of IDO1, D-1-methyl-tryptophan (D-1MT), effectively increased EpCAM/CD3-bispecific BiTE antibody MT110 efficacy against IDO1(hi)breast cancer via enhancing immune cells activity. Int Immunopharmacol, 2018,54:118–124
Ferrari F, Bellone S, Black J, et al. Solitomab, an EpCAM/CD3 bispecific antibody construct (BiTE (R)), is highly active against primary uterine and ovarian carcinosarcoma cell lines in vitro. J Exp Clin Cancer Res, 2015,34:123
Author information
Authors and Affiliations
Corresponding authors
Additional information
Conflict of Interest Statement
The authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
Author Shi-xuan WANG is a member of the Editorial Board for Current Medical Science. The paper was handled by other editors and has undergone rigorous peer review process. Author Shi-xuan WANG was not involved in the journal’s review of, or decisions related to, this manuscript.
This work was supported by the National Key Research & Development Program of China (No. 2021YFC2701402).
Rights and permissions
About this article
Cite this article
Li, Yn., Li, Yy., Wang, Sx. et al. Efficacy of Bispecific Antibody Targeting EpCAM and CD3 for Immunotherapy in Ovarian Cancer Ascites: An Experimental Study. CURR MED SCI 43, 539–550 (2023). https://doi.org/10.1007/s11596-023-2753-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-023-2753-2