Abstract
Objective
The occurrence and development of inflammation are closely correlated to the polarization of macrophages. All-trans retinoic acid (ATRA) has been proven to promote the polarization of macrophages from M1 to M2, but this lacks an effective carrier to participate in the biological response. The present study aims to determine whether retinoic acid-incorporated glycol chitosan (RA-GC) nanoparticles can regulate macrophage polarization in Porphyromonas gingivalis-lipopolysaccharide (Pg-LPS)-induced inflammation.
Methods
Mouse 264.7 cell lines were treated with 1 µg/mL Pg-LPS to induce inflammation. After the effects of ATRA and RA-GC on the activity of macrophages were detected by CCK-8 assay, cells induced with Pg-LPS were assigned to the blank control group (GC) nanoparticles without ATRA, and experimental groups (GC nanoparticles loaded with different concentrations of ATRA: 1, 10 and 100 µg/mL). The effects of RA-GC on inflammatory cytokines tumor necrosis factor-α, interleukin (IL)-10 and IL-12 in macrophages were detected by enzyme-linked immunosorbent assay (ELISA). Subsequently, the effects of GC nanoparticles loaded with/without ATRA on macrophage polarization in an inflammatory environment were detected by RT-PCR and Western blotting.
Results
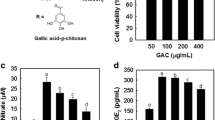
The results revealed that RA-GC had no significant effect on macrophage activity. However, RA-GC could effectively inhibit the Pg-LPS-induced inflammatory factor expression in macrophages. Meanwhile, the experimental results confirmed that RA-GC could downregulate the expression of inducible nitric oxide synthase (iNOS) (a marker of M1 macrophages) and upregulate the expression of mannose receptor and Arginase-1 (a marker of M2 macrophages) in a dose-dependent manner.
Conclusion
The present study confirms that RA-GC can promote the M2 polarization of macrophages in an inflammatory environment, and proposes this as a promising target for the clinical treatment of Pg-LPS-related diseases.
Similar content being viewed by others
References
Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol, 2005, 5(12): 953–964
Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep, 2014, 6: 13
Shrivastava R, Shukla N. Attributes of alternatively activated (M2) macrophages. Life Sci, 2019,(224):222-231
Spiller KL, Koh TJ. Macrophage-based therapeutic strategies in regenerative medicine. Adv Drug Deliv Rev, 2017, 122: 74–83
Martinez FO, Sica A, Mantovani A, et al. Macrophage activation and polarization. Front Biosci, 2008, 13: 453–461
Mirza R, Koh TJ. Dysregulation of monocyte/macrophage phenotype in wounds of diabetic mice. Cytokine, 2011, 56(2): 256–264
Kigerl KA, Gensel JC, Ankeny DP, et al. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci, 2009, 29(43): 13435–13444
Hu G, Guo M, Xu J, et al. Nanoparticles Targeting Macrophages as Potential Clinical Therapeutic Agents Against Cancer and Inflammation. Front Immunol, 2019, 10: 1998
Yuan Y, Long L, Liu J, et al. The double-edged sword effect of macrophage targeting delivery system in different macrophage subsets related diseases. J Nanobiotechnol, 2020, 18(1): 168
Altucci L, Leibowitz MD, Ogilvie KM, et al. RAR and RXR modulation in cancer and metabolic disease. Nat Rev Drug Disc, 2007, 6(10): 793–810
Dar PA, Singh LR, Kamal MA, et al. Unique Medicinal Properties of Withania somnifera: Phytochemical Constituents and Protein Component. Curr Pharm Design, 2016, 22(5): 535–540
Yan Q, Li Y, Cheng N, et al. Effect of retinoic acid on the function of lipopolysaccharide-stimulated bone marrow stromal cells grown on titanium surfaces. Inflamm Res, 2015, 64(1): 63–70
Zhong C, Pu L, Fang M, et al. ATRA Regulates Innate Immunity in Liver Ischemia/Reperfusion Injury via RARalpha/Akt/Foxo1 Signaling. Biol Pharm Bull, 2018, 41(4): 530–535
Lee B, Wu CY, Lin YW, et al. Synergistic activation of Arg1 gene by retinoic acid and IL-4 involves chromatin remodeling for transcription initiation and elongation coupling. Nucleic Acids Res, 2016, 44(16): 7568–7579
Vellozo NS, Pereira-Marques ST, Cabral-Piccin MP, et al. All-Trans Retinoic Acid Promotes an M1- to M2-Phenotype Shift and Inhibits Macrophage-Mediated Immunity to Leishmania major. Front Immunol, 2017, 8: 1560
Szuts EZ, Harosi FI. Solubility of retinoids in water. Arch Biochem Biophys, 1991, 287(2): 297–304
Conley BA, Egorin MJ, Sridhara R, et al. Phase I clinical trial of all-trans-retinoic acid with correlation of its pharmacokinetics and pharmacodynamics. Cancer Chemoth Pharmacol, 1997, 39(4): 291–299
Frankel SR, Eardley A, Lauwers G, et al. The “retinoic acid syndrome” in acute promyelocytic leukemia. Ann Intern Med, 1992, 117(4): 292–296
Kim IY, Seo SJ, Moon HS, et al. Chitosan and its derivatives for tissue engineering applications. Biotechnol Adv, 2008, 26(1): 1–21
Vasconcelos DP, Fonseca AC, Costa M, et al. Macrophage polarization following chitosan implantation. Biomaterials, 2013, 34(38): 9952–9959
Bhattarai N, Ramay HR, Gunn J, et al. PEG-grafted chitosan as an injectable thermosensitive hydrogel for sustained protein release. J Control Release, 2005, 103(3): 609–624
Park JS, Koh YS, Bang JY, et al. Antitumor effect of all-trans retinoic acid-encapsulated nanoparticles of methoxy poly(ethylene glycol)-conjugated chitosan against CT-26 colon carcinoma in vitro. J Pharm Sci, 2008, 97(9): 4011–4019
Chung KD, Jeong YI, Chung CW, et al. Anti-tumor activity of all-trans retinoic acid-incorporated glycol chitosan nanoparticles against HuCC-T1 human cholangiocarcinoma cells. Int J Pharm, 2012, 422(1–2): 454–461
Jeong YI, Kim SH, Jung TY, et al. Polyion complex micelles composed of all-trans retinoic acid and poly (ethylene glycol)-grafted-chitosan. J Pharm Sci, 2006, 95(11): 2348–2360
Thunemann AF, Beyermann J, Kukula H. Poly(ethylene oxide)-b-poly(l-lysine) Complexes with Retinoic Acid. Macromolecules, 2000, 33(16): 5906–5911
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that there are no conflicts of interest in this work.
Additional information
The study was supported by the Natural Science Foundation of Fujian Province (No. 2018J01245).
Rights and permissions
About this article
Cite this article
He, Tr., Tang, Xy., Yan, Q. et al. All-trans Retinoic Acid-incorporated Glycol Chitosan Nanoparticles Regulate Macrophage Polarization in Pg-LPS-Induced Inflammation. CURR MED SCI 42, 974–980 (2022). https://doi.org/10.1007/s11596-022-2602-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-022-2602-8