Abstract
Objective
Intracerebral hemorrhage (ICH) refers to predominant, sporadic, and non-traumatic bleeding in the brain parenchyma. The PI3K/AKT/mTOR signaling pathway is an important signal transduction pathway regulated by enzyme-linked receptors and has many biological functions in mammals. It plays a key role in neuronal metabolism, gene expression regulation, and tissue homeostasis in the healthy and diseased brain.
Methods
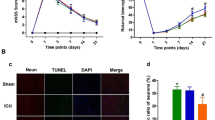
In the present study, the role of the PI3K/AKT/mTOR pathway inhibitor chrysophanol (CPH) (10 mg/kg and 20 mg/kg, orally) in the improvement of ICH-associated neurological defects in rats was investigated. Autologous blood (20 µL/5 min/unilateral/intracerebroventricular) mimics ICH-like defects involving cellular and molecular dysfunction and neurotransmitter imbalance. The current study also included various behavioral assessments to examine cognition, memory, and motor and neuromuscular coordination. The protein expression levels of PI3K, AKT, and mTOR as well as myelin basic protein and apoptotic markers, such as Bax, Bcl-2, and caspase-3, were examined using ELISA kits. Furthermore, the levels of various neuroinflammatory cytokines and oxidative stress markers were assessed. Additionally, the neurological severity score, brain water content, gross brain pathology, and hematoma size were used to indicate neurological function and brain edema.
Results
CPH was found to be neuroprotective by restoring neurobehavioral alterations and significantly reducing the elevated PI3K, AKT, and mTOR protein levels, and modulating the apoptotic markers such as Bax, Bcl-2, and caspase-3 in rat brain homogenate. CPH substantially reduced the inflammatory cytokines like interleukin (IL)-1β, IL-6, and tumor necrosis factor-α. CPH administration restored the neurotransmitters GABA, glutamate, acetylcholine, dopamine, and various oxidative stress markers.
Conclusion
Our results show that CPH may be a promising therapeutic approach for overcoming neuronal damage caused by the overexpression of the PI3K/AKT/mTOR signaling pathway in ICH-induced neurological dysfunctions in rats.
Similar content being viewed by others
References
Qureshi AI, Mendelow AD, Hanley DF. Intracerebral haemorrhage. Lancet, 2009,373(9675):1632–44.
Copotoiu R, Cinca E, Collange O et al, Pathophysiology ofhemorragic shock. Transfus Clin Biol, 2016,23(4):222–228
Qureshi AI, Suri MF, Ostrow PT, et al. Apoptosis as a form of cell death in intracerebral hemorrhage. Neurosurgery, 2003,52(5):1041–1048
Chaudhry BZ, Manno EM. Intracerebral Hemorrhage: An Overview of Etiology, Pathophysiology, Clinical Presentation, and Advanced Treatment Strategies. InManagement of Bleeding Patients, Springer, Cham, 2016:171–183
Duan X, Wen Z, Shen H, et al. Intracerebral hemorrhage, oxidative stress, and antioxidant therapy. Oxid Med Cell Longev, 2016:1203285
Graham DI, McIntosh TK, Maxwell WL, et al. Recent advances in neurotrauma. J Neuropathol Exp Neurol, 2000,59(8):641–651
Kalogeris T, Baines CP, Krenz M, et al. Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol, Academic Press, 2012:229–317
Keep RF, Hua Y, Xi G. Intracerebralhaemorrhage: mechanisms of injury and therapeutic targets. Lancet Neurol, 2012,11(8):720–731
Schlunk F, Greenberg SM. The pathophysiology of intracerebral hemorrhage formation and expansion.. Transl Stroke Res, 2015,6(4):257–263
Aronowski J, Hall CE. New horizons for primary intracerebral hemorrhage treatment: experience from preclinical studies. Neurol Res, 2005,27(3):268–279
Hua Y, Wu J, Keep RF, et al. Tumor necrosis factor-α increases in the brain after intracerebral hemorrhage and thrombin stimulation. Neurosurgery, 2006,58(3):542–550
Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol, 2006, 5(1):53–63
Fisher JC, Pry RH. A simple substitution model of technological change. Technological forecasting and social change,1971,3:75–88
Zipfel GJ, Han H, Ford AL, et al. Cerebral amyloid angiopathy: progressive disruption of the neurovascular unit. Stroke, 2009,40(3 Suppl):S16–S19
Wang HB, Wu QJ, Zhao SJ, et al. Early High Cerebrospinal Fluid Glutamate: A Potential Predictor for Delayed Cerebral Ischemia after Aneurysmal Subarachnoid Hemorrhage. ACS omega, 2020,5(25): 15 385–15 389
Qureshi AI, Mendelow AD, Hanley DF. Intracerebral-haemorrhage. Lancet, 2009,373(9675):1632–1644
Magistris F, Bazak S, Martin J. Intracerebral hemorrhage: pathophysiology, diagnosis and management. MUMJ, 2013,10(1):15–22
Deinsberger W, Vogel J, Kuschinsky W, et al. Experimental intracerebral hemorrhage: description of a double injection model in rats. Neurol Res, 1996,18(5): 475–477
Lee JY, Sagher O, Keep R, et al. Comparison of experimental rat models of early brain injury after subarachnoid hemorrhage. Neurosurgery, 2009,65(2): 331–343
Liu H, Sun X, Zou W, et al. Scalp acupuncture attenuates neurological deficits in a rat model of hemorrhagic stroke. Complement Ther Med, 2017,32:85–90
Tao C, Keep RF, Xi G, et al. CD47 blocking antibody accelerates hematoma clearance after intracerebral hemorrhage in aged rats. Transl Stroke Res, 2020,11(3): 541–551
Chen-Roetling J, Kamalapathy P, Cao Y et al. Astrocyte heme oxygenase-1 reduces mortality and improves outcome after collagenase-induced intracerebral hemorrhage. Neurobiol Dis, 2017, 102:140–146
Krafft PR, Rolland WB, Duris K, et al. Modeling intracerebral hemorrhage in mice: injection of autologous blood or bacterial collagenase. J Vis Exp, 2012, (67):e4289
Neri LM, Borgatti P, Capitani S, et al. The nuclear phosphoinositide 3-kinase/AKT pathway: a new second messenger system. Biochim Biophys Acta, 2002, 1584(2–3):73–80
Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet, 2006,7(8):606–619
Sharma A, Mehan S. Targeting PI3K-AKT/mTOR signaling in the prevention of autism. Neurochem Int, 2021,147:105067
Mammana S, Bramanti P, Mazzon E, et al. Preclinical evaluation of the PI3K/Akt/mTOR pathway in animal models of multiple sclerosis. Oncotarget, 2018,9(9): 8263–8277
Yudushkin I. Getting the Akt together: guiding intracellular Akt activity by PI3K. Biomolecules, 2019, 9(2):67
Jaworski J, Spangler S, Seeburg DP, et al. Control of dendritic arborization by the phosphoinositide-3′-kinase-Akt-mammalian target of rapamycin pathway. J Neurosci, 2005,25(49):11 300–11 312
Akiyama H, Kamiguchi H. Phosphatidylinositol 3-kinase facilitates microtubule-dependent membrane transport for neuronal growth cone guidance. J Biol Chem, 2010,285(53):41740–41748
Mammana S, Bramanti P, Mazzon E, et al. Preclinical evaluation of the PI3K/Akt/mTOR pathway in animal models of multiple sclerosis. Oncotarget, 2018,9(9):8263
Zhang W, Khatibi NH, Yamaguchi-Okada M, et al. Mammalian target of rapamycin (mTOR) inhibition reduces cerebral vasospasm following a subarachnoid hemorrhage injury in canines. Exp Neurol, 2012,233(2): 799–806
Chen A, Xiong LJ, Tong Y, et al. Neuroprotective effect of brain-derived neurotrophic factor mediated by autophagy through the PI3K/Akt/mTOR pathway. Mol Med Rep, 2013,8(4):1011–1016
Rivière JB, Mirzaa GM, O’Roak BJ, et al. De novo germline and postzygotic mutations in AKT3, PIK3R2 and PIK3CA cause a spectrum of related megalencephaly syndromes. Nat Genet, 2012,44(8):934–940
Xiao Z, Peng J, Yang L, et al. Interleukin-1β plays a role in the pathogenesis of mesial temporal lobe epilepsy through the PI3K/Akt/mTOR signaling pathway in hippocampal neurons. J Neuroimmunol, 2015,282:110–117
Brandt C, Hillmann P, Noack A, et al. The novel, catalytic mTORC1/2 inhibitor PQR620 and the PI3K/mTORC1/2 inhibitor PQR530 effectively cross the blood-brain barrier and increase seizure threshold in a mouse model of chronic epilepsy. Neuropharmacology, 2018,140:107–120
Ali T, Kim T, Rehman SU, et al. Natural dietary supplementation of anthocyanins via PI3K/Akt/Nrf2/HO-1 pathways mitigate oxidative stress, neurodegeneration, and memory impairment in a mouse model of Alzheimer’s disease. Mol Neurobiol, 2018, 55(7):6076–6093
Hodges SL, Reynolds CD, Smith GD, et al. Molecular interplay between hyperactive mammalian target of rapamycin signaling and Alzheimer’s disease neuropathology in the NS-Pten knockout mouse model. Neuro Report, 2018,29(13):1109–1113
Giacoppo S, Pollastro F, Grassi G, et al. Target regulation of PI3K/Akt/mTOR pathway by cannabidiol in treatment of experimental multiple sclerosis. Fitoterapia, 2017,116:77–84
Abd-Elrahman KS, Ferguson SS. Modulation of mTOR and CREB pathways following mGluR5 blockade contribute to improved Huntington’s pathology in zQ 175 mice. Mol Brain, 2019,12(1):1–9
Chen Y, Zheng X, Wang Y, et al. Effect of PI3K/Akt/mTOR signaling pathway on JNK3 in Parkinsonian rats. Exp Ther Med, 2019,17(3):1771–1775
Daniel PM, Filiz G, Brown DV, et al. PI3K activation in neural stem cells drives tumorigenesis which can be ameliorated by targeting the cAMP response element binding protein. Neuro Oncol, 2018,20(10):1344–55.
Singh D, Rawat MS, Semalty A, et al. Chrysophanol-phospholipid complex. J Therm Anal Calorim, 2013, 111(3):2069–2077
Lu CC, Yang JS, Huang AC, et al. Chrysophanol induces necrosis through the production of ROS and alteration of ATP levels in J5 human liver cancer cells. Mol Nutr Food Res, 2010,54(7):967–976
Zhang J, Yan C, Wang S, et al. Chrysophanol attenuates lead exposure-induced injury to hippocampal neurons in neonatal mice. Neural Regen Res, 2014,9(9):924
Mishra V. Potent gastroprotective effect chrysophanol and emodin from Rheum emodi via H+ K+ Atpase inhibition and increasing the Pge2 level in rats. Nat Prod Indian J, 2016,12:1–2
Zhang J, Kang H, Wang L, et al. Chrysophanol ameliorates high-fat diet-induced obesity and inflammation in neonatal rats. Pharmazie, 2018,73(4):228–233
Chae U, Min JS, Leem HH, et al. Chrysophanol suppressed glutamate-induced hippocampal neuronal cell death via regulation of dynamin-related protein 1-dependent mitochondrial fission. Pharmacology, 2017,100(3–4):153–160
Ye T, Li X, Zhou P, et al. Chrysophanol improves memory ability of d-galactose and Aβ 25–35 treated rat correlating with inhibiting tau hyperphosphorylation and the CaM-CaMKIV signal pathway in hippocampus. 3 Biotech, 2020,10(3):1–8
Lee MJ, Choi JH, Lee SJ, et al. Oriental medicine Samhwangsasim-tang alleviates experimental autoimmune encephalomyelitis by suppressing Th1 cell responses and upregulating Treg cell responses. Front Pharmacol, 2017,8:192
Mamik MK, Power C. Immune Sensors and Effectors of Health and Disease. In: Neuroimmune Pharmacology, Springer: Cham, 2017:93–105.
Zhao Y, Huang Y, Fang Y, et al. Chrysophanol attenuates nitrosative/oxidative stress injury in a mouse model of focal cerebral ischemia/reperfusion. J Pharmacol Sci, 2018,138(1):16–22
Zhao Y, Fang Y, Li J, et al. Neuroprotective effects of chrysophanol against inflammation in middle cerebral artery occlusion mice. NeurosciLett, 2016,630:16–22
Jiang W, Zhou R, Li P, et al. Protective effect of chrysophanol on LPS/d-GalN-induced hepatic injury through the RiP140/NF-κB pathway. RSC advances, 2016,6(44):38 192–38 200
Yusuf MA, Singh BN, Sudheer S, et al. Chrysophanol: a natural anthraquinone with multifaceted biotherapeutic potential. Biomolecules, 2019,9(2):68
Jeong HJ, Kim HY, Kim HM. Molecular mechanisms of anti-inflammatory effect of chrysophanol, an active component of AST2017-01 on atopic dermatitis in vitro models. Int Immunopharmacol, 2018,54:238–244
Hao Z, Liu M, Counsell C, et al. Fibrinogen depleting agents for acute ischaemic stroke. Cochrane Database Syst Rev, 2012(3):CD000091
Lim W, Yang C, Bazer FW, et al. Chrysophanol induces apoptosis of choriocarcinoma through regulation of ROS and the AKT and ERK1/2 pathways. J Cell Physiol, 2017,232(2):331–339
Chu X, Zhou S, Sun R, et al. Chrysophanol relieves cognition deficits and neuronal loss through inhibition of inflammation in diabetic mice. Neurochem Res, 2018,43(4):972–983
Rajdev K, Siddiqui EM, Jadaun KS, et al. Neuroprotective potential of solanesol in a combined model of intracerebral and intraventricular hemorrhage in rats. IBRO Rep, 2020,8:101–114
MehmoodSiddiqui E, Mehan S, Upadhayay S, et al. Neuroprotective efficacy of 4-hydroxyisoleucine in experimentally induced intracerebral hemorrhage. Saudi J Biol Sci, 2021,28(11):6417–6431
Singh A, Upadhayay S, Mehan S, et al. Inhibition of c-JNK/p38MAPK signaling pathway by Apigenin prevents neurobehavioral and neurochemical defects in ethidium bromide-induced experimental model of multiple sclerosis in rats: Evidence from CSF, blood plasma and brain samples. Phytomed Plus, 2021,1(4): 100139
Li M, Xia M, Chen W, et al. Lithium treatment mitigates white matter injury after intracerebral hemorrhage through brain-derived neurotrophic factor signaling in mice. Transl Res, 2020,217:61–74
Verma L, Sakir M, Singh N, et al. Development of phase change solutions for ophthalmic drug delivery based on ion activated and pH induced polymers. Int J Pharm Prof Res, 2010,1(2):127–134
Rynkowski MA, Kim GH, Komotar RJ, et al. A mouse model of intracerebral hemorrhage using autologous blood infusion. Nat Protoc, 2008,3(1):122
Xue M, Del Bigio MR. Intracerebral injection of autologous whole blood in rats: time course of inflammation and cell death. Neurosci Lett, 2000,283(3): 230–232
Bala R, Khanna D, Mehan S, et al. Experimental evidence for the potential of lycopene in the management of scopolamine induced amnesia. RSC Adv, 2015,5(89):72881–72892
Alam M, Minz E, Yadav R, et al, Neuroprotective potential of adenylcyclase/cAMP/CREB and mitochondrial CoQ10 activator in amyotrophic lateral sclerosis rats. Curr Bioactive Compounds, 2021(5):53–69
Sharma R, Rahi S, Mehan S. Neuroprotective potential of solanesol in intracerebroventricular propionic acid induced experimental model of autism: Insights from behavioral and biochemical evidence. Toxicol Rep, 2019,6:1164–1175
Mehan S, Monga V, Rani M, et al. Neuroprotective effect of solanesol against 3-nitropropionic acid-induced Huntington’s disease-like behavioral, biochemical, and cellular alterations: Restoration of coenzyme-Q10-mediated mitochondrial dysfunction. Indian J Pharmacol, 2018,50(6):309
Brivio P, Sbrini G, Riva MA, et al. Acute stress induces cognitive improvement in the novel object recognition task by transiently modulating Bdnf in the prefrontal cortex of male rats. Cell Mol Neurobiol, 2020,40(6):1037–1047
Cui J, Cui C, Cui Y, et al. Bone marrow mesenchymal stem cell transplantation increases GAP-43 expression via ERK1/2 and PI3K/Akt pathways in intracerebral hemorrhage. Cell Physiol Biochem, 2017,42(1):137–144
Wu Y, Wang L, Hu K, et al. Mechanisms and therapeutic targets of depression after intracerebral hemorrhage. Front Psychiatry, 2018,9:682
Zhang CY, Ren XM, Li HB, et al. Effect of miR-130a on neuronal injury in rats with intracranial hemorrhage through PTEN/PI3K/AKT signaling pathway. Eur Rev Med Pharmacol Sci, 2019,23:4890–4897
Tiwari A, Khera R, Rahi S, et al. Neuroprotective Effect of a-Mangostin in the Ameliorating Propionic Acid-Induced Experimental Model of Autism in Wistar Rats. Brain Sci, 2021,11(3):288
Kumar N, Sharma N, Khera R, et al. Guggulsterone ameliorates ethidium bromide-induced experimental model of multiple sclerosis via restoration of behavioral, molecular, neurochemical and morphological alterations in rat brain. Metab Brain Dis, 2021,36(5):911–925
Minj E, Upadhayay S, Mehan S. Nrf2/HO-1 Signaling Activator Acetyl-11-keto-beta Boswellic Acid (AKBA)-Mediated Neuroprotection in Methyl Mercury-Induced Experimental Model of ALS. Neurochem Res, 2021, 46(11):2867–2884
Zeng QH, Jiang YL, Wang Y, et al. The correlation between noradrenaline and acetylcholine levels and autonomic nervous system dysfunction in patients with stroke-associated pneumonia. Int J Clin Exp Med, 2017,10(10):14761–14769.
Jamwal S, Kumar P. Spermidine ameliorates 3-nitropropionic acid (3-NP)-induced striatal toxicity: possible role of oxidative stress, neuroinflammation, and neurotransmitters. Physiol Behav, 2016,155:180–187
Sharma N, Upadhayay S, Shandilya A, et al. Neuroprotection by solanesol against ethidium bromide-induced multiple sclerosis-like neurobehavioral, molecular, and neurochemical alterations in experimental rats. Phytomed Plus, 2021,1(4):100051
Mehan S, Parveen S, Kalra S. Adenylcyclase activator forskolin protects against Huntington’s disease-like neurodegenerative disorders. Neural Regen Res, 2017, 12(2):290
Duggal P, Jadaun K.S, Siqqiqui, et al. Investigation of low dose cabazitaxel potential as microtubule stabilizer in experimental model of Alzheimer’s disease: Restoring neuronal cytoskeleton. Curr Alzheimer Res, 2020,17(7),601–615
Singh N, Bansal Y, Bhandari R, et al. Naringin reverses neurobehavioral and biochemical alterations in intracerebroventricular collagenase-induced intracerebral hemorrhage in rats. Pharmacology, 2017,100(3–4): 172–187
Rahi S, Gupta R, Sharma A, et al. Smo-Shh signaling activator purmorphamine ameliorates neurobehavioral, molecular, and morphological alterations in an intracerebroventricular propionic acid-induced experimental model of autism. Hum Exp Toxicol, 2021,40(11): 1880–1898
Lee KY, Kim DI, Kim SH, et al. Sequential combination of intravenous recombinant tissue plasminogen activator and intra-arterial urokinase in acute ischemic stroke. AJNR Am J Neuroradiol, 2004,25(9):1470–1475
Wang T, Xu L, Gao L, et al. Paeoniflorin attenuates early brain injury through reducing oxidative stress and neuronal apoptosis after subarachnoid hemorrhage in rats. Metab Brain Dis, 2020,35(6):959–970
Dudi R, Mehan S. Neuroprotection of brain permeable Forskolin ameliorates behavioral, biochemical and histopatho-logical alterations in rat model of intracerebral hemorrhage. Pharmaspire, 2018,10:68–86
Liu L, Wang S, Xu R, et al. Experimental intracerebralhaemorrhage: description of a semi-coagulated autologous blood model in rats. Neurol Res, 2015,37(10):874–879
Terai K, Suzuki M, Sasamata M, et al. Amount of bleeding and hematoma size in the collagenase-induced intracerebral hemorrhage rat model. Neurol Res, 2003, 28(5):779–785
Wen Q, Mei L, Ye S, et al. Chrysophanol demonstrates anti-inflammatory properties in LPS-primed RAW 264.7 macrophages through activating PPAR-γ. Int Immunopharmacol, 2018,56:90–97
Van Asch CJ, Luitse MJ, Rinkel GJ, et al. Incidence, case fatality, and functional outcome of intracerebralhaemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol, 2010,9(2):167–176
Buitendag JJ, Kong VY, Bruce JL, et al. The spectrum and outcome of paediatric traumatic brain injury in KwaZulu-Natal Province, South Africa has not changed over the last two decades. S Afr Med J, 2017,107(9):777–780
Lu Q, Huang L, Zhu GQ. A rat model of intracerebral hemorrhage induced by collagenase IV. Bio-protocol, 2015,5(14):e1541–e1541
Ostrowski RP, Colohan AR, Zhang JH. Molecular mechanisms of early brain injury after subarachnoid hemorrhage. Neurol Res, 2006,28(4):399–414
Zhang HB, Tu XK, Chen Q, et al. Propofol Reduces Inflammatory Brain Injury after Subarachnoid Hemorrhage: Involvement of PI3K/Akt Pathway. J Stroke Cerebrovasc Dis, 2019,28(12):104375
Zhang J, Kang H, Wang L, et al. Chrysophanol ameliorates high-fat diet-induced obesity and inflammation in neonatal rats. Pharmazie, 2018,73(4):228–233
Gong C, Boulis N, Qian J, et al. Intracerebral hemorrhage-induced neuronal death. Neurosurgery, 2001,48(4):875–883
Duan X, Wen Z, Shen H, et al. Intracerebral hemorrhage, oxidative stress, and antioxidant therapy. Oxid Med Cell Longev, 2016:1203285
Kale A, Pişkin Ö, Baş, Y, et al. Neuroprotective effects of Quercetin on radiation-induced brain injury in rats. J Radiat Res, 2018,59(4):404–410
Aladag MA, Turkoz Y, Parlakpinar H, et al. Nebivolol attenuates cerebral vasospasm both by increasing endothelial nitric oxide and by decreasing oxidative stress in an experimental subarachnoid haemorrhage. Br J Neurosurg, 2017,31(4):439–445
Galho AR, Cordeiro MF, Ribeiro SA, et al. Protective role of free and quercetin-loaded nanoemulsion against damage induced by intracerebralhaemorrhage in rats. Nanotechnology, 2016,27(17):175101
Buss L, Fisher E, Hardy J, et al. Intracerebralhaemorrhage in Down syndrome: protected or predisposed? F1000Res, 2016,5:F1000
Fathimoghadam H, Farbod Y, Ghadiri A, et al. Moderating effects of crocin on some stress oxidative markers in rat brain following demyelination with ethidium bromide. Heliyon, 2019,5(2):e01213
Shi K, Tian DC, Li ZG, et al. Global brain inflammation in stroke. Lancet Neurol, 2019,18(11):1058–1066
Zhao H, Pan P, Yang Y, et al. Endogenous hydrogen sulphide attenuates NLRP3 inflammasome-mediated neuroinflammation by suppressing the P2X7 receptor after intracerebralhaemorrhage in rats. J Neuroinflammation, 2017,14(1):163
Lan X, Han X, Li Q, et al. Modulators of microglial activation and polarization after intracerebralhaemorrhage. Nat Rev Neurol, 2017,13(7):420
Cui J, Cui C, Cui Y, et al. Bone marrow mesenchymal stem cell transplantation increases GAP-43 expression via ERK1/2 and PI3K/Akt pathways in intracerebral hemorrhage. Cell Physiol Biochem, 2017,42(1):137–44.
Wu Y, Wang L, Hu K, et al. Mechanisms and therapeutic targets of depression after intracerebral hemorrhage. Front Psychiatry, 2018,9:682
Zhang L, Plotkin RC, Wang G, et al. Cholinergic augmentation with donepezil enhances recovery in short-term memory and sustained attention after traumatic brain injury. Arch Phys Med Rehabil, 2004,85(7):1050–1055
Chen B, Zhao Y, Li W, et al. Echinocystic acid provides a neuroprotective effect via the PI3K/AKT pathway in intracerebral haemorrhage mice. Ann Transl Med, 2020, 8(1):6
Chiang MF, Chiu WT, Lin FJ, et al. Multiparametric analysis of cerebral substrates and nitric oxide delivery in cerebrospinal fluid in patients with intracerebral haemorrhage: correlation with hemodynamics and outcome. Acta Neurochir (Wien), 2006,148(6):615–621
Wang J, Rogove AD, Tsirka AE, et al. Protective role of tuftsin fragment 1–3 in an animal model of intracerebral hemorrhage. Ann Neurol, 2003,54:655–664
Park S, Lim W, Song G. Chrysophanol selectively represses breast cancer cell growth by inducing reactive oxygen species production and endoplasmic reticulum stress via AKT and mitogen-activated protein kinase signal pathways. Toxicol Appl Pharmacol, 2018,360: 201–211
Zhang L, Plotkin RC, Wang G, et al. Cholinergic augmentation with donepezil enhances recovery in short-term memory and sustained attention after traumatic brain injury. Arch Phys Med Rehabil, 2004,85(7),1050–1055
Myhrer T. Neurotransmitter systems involved in learning and memory in the rat: a meta-analysis based on studies of four behavioral tasks. Brain Res Brain Res Rev, 2003,41(2–3):268–287
Su S, Wu J, Gao Y, et al. The pharmacological properties of chrysophanol, the recent advances. Biomed Pharmacother, 2020,125:110002
Acknowledgments
The authors express their gratitude to Chairman. Mr. Parveen Garg and Director, Dr. G. D. Gupta, ISF College of Pharmacy, Moga (Punjab), India, for their great support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Jadaun, K.S., Mehan, S., Sharma, A. et al. Neuroprotective Effect of Chrysophanol as a PI3K/AKT/mTOR Signaling Inhibitor in an Experimental Model of Autologous Blood-induced Intracerebral Hemorrhage. CURR MED SCI 42, 249–266 (2022). https://doi.org/10.1007/s11596-022-2496-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-022-2496-x