Summary
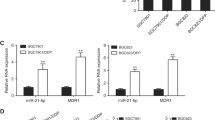
In order to investigate the effects of vector-based hairpin small interference RNA (shRNA) on the reversal of multi-drug resistance (mdr) of A2780/Taxol cells, a novel vector pEGFP-H1/mdr1 containing mdr1-shRNA targeting at position 2943–2963 of mdr1 was designed and synthesized. Subsequently, A2780/Taxol cells were transfected with pEGFP-H1/mdr1, and the expression of mdr1 mRNA and P-gp was detected by using RT-PCR and Western blot respectively. MTT was used to measure the 50% inhibition concentration (IC50) of Taxol to A2780/Taxol cells. The results showed that at the 24th and 48th h after transfection, the expression of mdr1 mRNA was decreased to (52.1±1.0)% and (0.01±1.7)%, and that of P-gp decreased to (88.3±2.1)% and 0%, respectively. At the 48th h after transfection, the relative reversal rate of A2780/Taxol cells to Taxol was 69.54%. In vivo, the nude mice xenografts were injected with pEGFP-H1/mdr1, and then administrated Taxol. The tumor volume in pEGFP-H1/mdr1-transfected group was significantly reduced as compared with that in blank control group or pEGFP-H1-transfected group (807.20±103.16 vs 1563.78±210.54 or 1480.78±241.24 mm3, both P<0.01). These results suggested that transfection of pEGFP-H1/mdr1 could efficiently down-regulate the expression of mdr1 mRNA and P-gp in A2780/Taxol cells, and effectively restore the sensitivity of A2780/Taxol cells to Taxol both in vitro and in vivo.
Similar content being viewed by others
References
Thomas H, Coley HM. Overcoming multidrug resistance in cancer: an update on the clinical strategy of inhibiting p-glycoprotein. Cancer Control, 2003,10(2): 159–165
Loo TW, Clarke DM. Recent progress in understanding the mechanism of P-glycoprotein-mediated drug efflux. J Membr Biol, 2005,206(3):173–185
Yusuf RZ, Duan Z, Lamendola DE, et al. Paclitaxel resistance: molecular mechanisms and pharmacologic manipulation. Curr Cancer Drug Targets, 2003,3(1):1–19
Roninson IB, Chin JE, Choi KG, et al. Isolation of human mdr DNA sequences amplified in multidrug-resistant KB carcinoma cells. Proc Natl Acad Sci USA, 1986,83(12): 4538–4542
Masanek U, Stammler G, Volm M. Modulation of multidrug resistance in human ovarian cancer cell lines by inhibition of P-glycoprotein 170 and PKC isoenzymes with antisense oligonucleotides. J Exp Ther Oncol, 2002,2(1): 37–41
Nagata J, Kijima H, Hatanaka H, et al. Reversal of drug resistance using hammerhead ribozymes against multidrug resistance-associated protein and multidrug resistance 1 gene. Int J Oncol, 2002,21(5):1021–1026
Ramachandran C, Wellham LL. Effect of MDR1 phosphorothioate antisenseoligodeoxynucleotides in multidrug-resistant human tumor cell lines and xenografts. Anticancer Res, 2003,23(3B):2681–2690
Nieth C, Priebsch A, Stege A, et al. Modulation of the classical multidrug resistance (MDR) phenotype by RNA interference (RNAi). FEBS Lett, 2003,545(2–3):144–150
Hannon GJ, Rossi JJ. Unlocking the potential of the human genome with RNA interference. Nature, 2004, 431(7006):373–378
Luan YZ, Li L, Li D, et al. Development of five kinds of drug-resistant ovarian cancer cell lines and expression of partial resistance-related genes. Chin J Obstet Gynecol (Chinese), 2004,39(6):403–407
Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: Role of ATP-dependent transporters. Nat Rev Cancer, 2002,2(1):48–58
Schwarz DS, Hutvagner G, Du T, et al. Asymmetry in the assembly of the RNAi enzyme complex. Cell, 2003,115(2):199–208
Lage H. MDR1/P-glycoprotein (ABCB1) as target for RNA interference-mediated reversal of multidrug resistance. Curr Drug Targets, 2006, 7(7):813–821
Matsukura S, Jones PA, Takai D. Establishment of conditional vectors for hairpin siRNA knockdowns. Nucl Acids Res, 2003,31(15):e77
Stevenson M. Therapeutic potential of RNA interference. N Engl J Med, 2004,351(17):1772–1777
Sui G, Soohoo C, Affar EIB, et al. A DNA vector based RNAi technology to suppress gene expression in mammalian cells. PNAS, 2002,99(8):5515–5520
Elbashir SM, Lendeckel W, Tuschl T. RNA interference is mediated by 21- and 22-nucleotide RNAs. Genes Dev, 2001,15(2):188–200
Lu S, Wang X, Cai L, et al. Inhibition of MDR1 gene by hairpin siRNA to reversal of multi-drug resistance in ovarian cancer cells. Chin J Obstet Gynecol (Chinese), 2007,42(4):60–62
Pan GD, Yang JQ, Yan LN, et al. Reversal of multi-drug resistance by pSUPER-shRNA-mdr1 in vivo and in vitro. World J Gastroenterol, 2009,15(4): 431–440
Stein U, Walther W, Stege A, et al. Complete in vivo reversal of the multidrug resistance phenotype by Jet-injection of anti-MDR1 short hairpin RNA-encoding plasmid DNA. Molecular Therapy, 2008,16(1): 178–186
Lu S, Wang X, Xiao L, et al. Gene therapy for ovarian cancer using adenovirus-mediated transfer of cytosine deaminase gene and uracil phosphoribosyltransferase gene directed by MDR1 promoter. Cancer Biology Therapy, 2007,6(3):397–404
Yang Y, Wang Z, Li M, et al. Chitosan/pshRNA plasmid nanoparticles targeting MDR1 gene reverse paclitaxel resistance in ovarian cancer cells. J Huazhong Univ Sci Technolog Med Sci, 2009,29(2):239–242
Author information
Authors and Affiliations
Additional information
This project was supported by grants from National Natural Sciences Foundation of China (No. 30070786), Scientific Research Foundation of Hubei Health Department (No. JX2B17), and Key Technologies R&D Programme of Hubei Province, China (No. 2007AA301C20).
Rights and permissions
About this article
Cite this article
Lu, S., Huang, Q., Wang, Z. et al. Reversal of multi-drug resistance by vector-based-ShRNA-Mdr1 In Vitro and In Vivo . J. Huazhong Univ. Sci. Technol. [Med. Sci.] 29, 620–624 (2009). https://doi.org/10.1007/s11596-009-0517-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-009-0517-2