Summary
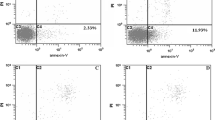
This study investigated the inhibitory effect of the extract of fungi of Huaier (EFH) on the growth of hepatocellular carcinoma (HCC) cells. Hep-G2 cells, a human HCC cell line, were cultured in DMEM containing 10% fetal bovine serum and treated with EFH of different concentrations (1, 2, 4, 8 mg/mL) for 24, 48 and 72 h respectively. The apoptosis rate of the cells was flow cytometrically measured. Thirty-six tumor-bearing New Zealand rabbits were randomly divided into 3 groups: group A (control group), in which the rabbits were infused with 0.2 mL/kg normal saline via the hepatic artery; group B (transhepatic artery chemoembolization [TACE] group), in which the rabbits were given lipiodol at 0.2 mL/kg plus MMC at 0.5 mg/kg via the hepatic artery; group C (TACE + EFH group ), in which EFH (500 mg/kg) were orally administered after TACE. Two weeks after TACE, the rabbits were sacrificed and the implanted tumors were sampled. The tumor volume and the necrosis rate were determined. The tumor tissues were immunohistochemically detected for the expressions of factor VIII, VEGF, P53, Bax and Bcl-2. The microvessel density (MVD) was calculated by counting the factor VIII-positive endothelial cells. Our results showed that after treatment with EFH, the apoptosis rate of Hep-G2 cells was enhanced in a concentration- and time-dependent manner. Two weeks after the treatment, the average tumor volume, the necrosis rate and the growth rate of the implanted tumor in group C were significantly different from those in groups A and B (P<0.05). MVD and VEGF expressions were significantly decreased in the group C when compared with those in groups B (P<0.05 for all). The Bax expression was weakest in group A and strongest in group C. The expressions of P53 and Bcl-2 were minimal in group C and maximal in group A. There were significant differences in the expressions of P53, Bax and Bcl-2 among the 3 groups (P<0.05 for all) and there was significant difference between group B and group C (P<0.05). It was concluded that EFH could suppress not only the growth of HCC cells but also tumor angiogenesis and it can induce the apoptosis of HCC cells. EFH serves as an alternative for the treatment of HCC.
Similar content being viewed by others
References
O’suilleabhain CB, Poon RT, Yong JL, et al. Factors predictive of 5-year survival after transarterial chemoembolization for inoperable hepatocellular carcinoma. Br J Surg, 2003,90(3):325–331
Liado L, Virgili J, Figueras J, et al. A prognostic index of the survival of patients with unresectable hepatocellular carcinoma after transcatheter arterial chemoembolization. Cancer, 2000,88(1):50–57
Guan YS, Yuan L. Interventional treatments for hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int, 2006, 5(4):495–500
Kong J, Feng GS, Xu LF, et a1. Experimental Methodology of Transhepatic Arterial Chemoembolization in Rabbits: A Comparative Study. J Clin Radiol (Chinese), 2003,22(3):244–247
Zhou CK, Liang HM, Li X, et al. Establishment of rabbit model bearing VX2 liver tumor experimentation and discussion of the selective hepatic arterial catheterization. J Intervent Radiol (Chinese), 2006,15(2):101–104
Park Y N, Kim Y B, Yang K M, et al. Increased expression of vascular endothelial growth factor and angiogenesis in the early stage of multistep hepatocarcinogenesis. Arch Pathol Lab Med, 2000,124(7):1061–1065
Weidner N, Semple JP, Welch WR, et al. Tumor angiogenesis and metastasis-correlation in invasive breast carcinoma. N Engl J Med, 1991,324(1):1–8
Xu GL, Jia WD, Ma JL, et al. Experimental study of extract of funji of huaier on angiogenesis in vitro. Chin Pharmacol Bull (Chinese), 2003,19(12):1410–1412
Huang T, Kong QZ, Lu HD, et al. Experimental study of extract of funji of huaier inducing apoptosis of the human adenocarcinoma of lung A549 cells. Chin J Tuberc Respir Dis (Chinese), 2001,24(8):487–488
Gerl R, Vaux DL. Apoptosis in the development and treatment of cancer. Carcinogenesis, 2005,26(2):263–270
Vaux D L. Early work on the function of Bcl-2, an interview with David Vaux. Cell Death Differ, 2004,11(11): 528–532
Tophkhane C, Yang S, Bales W, et al. Bcl-2 overexpression sensitizes MCF-7 cells to genistein by multiple mechanisms. Int J Oncol, 2007,31(4):867–874
Wei MC, Zong WX, Cheng EH, et al. Pro-apoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science, 2001,292(5517):727–730
Cory S, Adams JM. The Bcl-2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer, 2002,2(9): 647–656
Soini Y, Virkajarvi N, Lehto VP, et al. Hepatocellular carcinomas with a high proliferation index and a low degree of apoptosis and necrosis are associated with a shortened survival. Br J Cancer, 1996,73(9):1025–1030
Marsden VS, O’Connor L, O’Reilly LA, et al. Apoptosis initiated by Bcl-2-regulated caspase activation independently of the cytochrome c/Apaf-1/caspase-9 apoptosome. Nature, 2002,419(6907):634–637
Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature, 2005,438(7070):967–974
Dvorak HF, Brown LF, Detmar M, et al. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol, 1995,146(5):1029–1039
Yancopoulos GD, Davis S, Gale NW, et al. Vascular-specific growth factors and blood vessel formation. Nature, 2000,407(6801):242–248
Hasan J, Byers R, Jayson GC. Intra-tumoural microvessel density in human solid tumours. Br J Cancer, 2002,86(10): 1566–1577
Fox CH, Whalen G F, Sanders MM, et al. Angiogenesis in normal tissue adjacent to colon cancer. J Surg Oncol, 1998, 69(4):230–223
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ren, J., Zheng, C., Feng, G. et al. Inhibitory effect of extract of fungi of Huaier on hepatocellular carcinoma cells. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 29, 198–201 (2009). https://doi.org/10.1007/s11596-009-0212-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-009-0212-3