Abstract
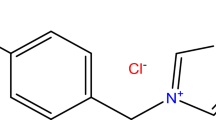
The inhibition effect of dimethylethanolamine (DMEA) and its composite with carboxylic acid was studied with the electrochemical tests. The experimental results indicate that DMEA is not a good inhibitor but the composite of DMEA with caprylic acid exhibits excellent inhibiting efficiency. The synergic mechanism of the organic corrosion inhibitors (OCIs) was studied with quantum chemical calculations. It is found that the DMEA forms a quaternary ammonium salt with the proton in carboxylic acid, and a cyclic complex formed between the salt and Fe may be responsible for the enhancement of inhibiting efficiency. The possible hydrogen bond formed between DMEA and carboxylic acid is not enough for the inhibiting effect. This work is helpful to proposing theoretical interpretation as well as developing a functional organic inhibitor to improve the durability of reinforced concrete contaminated with chloride.
Similar content being viewed by others
References
Elsener B, Buchler M, Bohni H. Corrosion Inhibitors for Steel in Concrete[C]. In: Proceedings of Eurocorr 97, Vol 1. Trondheim, Norway, European Federation of Corrosion, 1997. 469–474
Xu YM. Migrating Corrosion Inhibitor-A New Development of Corrosion Inhibitors for Steel Bar in Concrete (in Chinese)[J]. J. Chi. Ceram Soc., 2002, 30(1): 95–100
Doner A, Solmaz R, Ozcan M, et al. Experimental and Theoretical Studies of Thiazoles as Corrosion Inhibitors for Mild Steel in Sulphuric Acid Solution[J]. Corrosion Science, 2011, 53(9): 2 902–2 913
Flores EA, Olivares O, Likhanova NV, et al. Sodium Phthalamates as Corrosion Inhibitors for Carbon Steel in Aqueous Hydrochloric Acid Solution[J]. Corrosion Science, 2011, 53(12): 3 899–3 913
Gaidis JM. Chemistry of Corrosion Inhibitors[J]. Cement and Concrete Composites, 2004, 26(3): 181–189
Nmai CK. Multi-functional Organic Corrosion Inhibitor[J]. Cement and Concrete Composites, 2004, 26(3): 199–207
Soylev TA, Richardson MG. Corrosion Inhibitors for Steel in Concrete: State-of-the-art Report[J]. Construction and Building Materials, 2008, 22(4): 609–622
Ormellese M, Lazzari L, Goidanich S, et al. A Study of Organic Substances as Inhibitors for Chloride-induced Corrosion in Concrete[J]. Corrosion Science, 2009, 51(12): 2 959–2 968
Liu ZY, Miao CW, Sun W. Effect of Migratory Corrosion Inhibitors on the Durability of Chloride-contaminated Rereinforced Concretelong-term Effect, Morphological and Mechanisms Analysis[J]. J. Chi. Ceram Soc., 2010, 38: 1 321–1 327
Jamil HE, Shriri A, Boulif R, et al. Electrochemical Behaviour of Amino Alcohol-based Inhibitors Used to Control Corrosion of Reinforcing Steel[J]. Electrochimica Acta, 2004, 49(17): 2 753–2 760
Shimura T, Aramaki K. Preparation of a Self-assembled Monolayer on Iron by the Formation of a Covalent Bond between Carbon and Iron Atoms[J]. Corrosion Science, 2006, 48(11): 3 784–3 801
Lendvay-Győrik G, Mészáros G, Lengyel B, et al. Electrochemical and Quantum Chemical Studies on the Formation of Protective Films by Alkynols on Iron[J]. Corrosion Science, 2003, 45(8): 1 685–1 702
Jamil HE, Montemor MF, Boulif R, et al. An Electrochemical and Analytical Approach to the Inhibition Mechanism of an Aminoalcohol-based Corrosion Inhibitor for Reinforced Concrete[J]. Electrochimica Acta, 2003, 48(23): 3 509–3 518
Stern M, Geary AL. Electrochemical Polarization I. A Theoretical Analysis of the Shape of Polarization Curves[J]. Journal of the Electrochemical Society, 1957, 104(1): 56–63
Broomfield JP. Corrosion of Steel in Concrete: Understanding, Investigation and Repair[M]. 2nd Edition, Taylor & Francis Group, London and New York. 2007.
Frisch MJ, Trucks GW, Schlegel HB, et al. Gaussian 09, Revision A.02. Gaussian, Inc.: Wallingford, CT, 2009.
Becke AD. Density-functional Thermochemistry. III. The Role of Exact Exchange[J]. The Journal of Chemical Physics, 1993, 98(7): 5 648–5 652
Lee C, Yang W, Parr RG. Development of the Colle-Salvetti Correlation-energy Formula into a Functional of the Electron Density[J]. Physical Review B, 1988, 37(2): 785–789
Hay PJ, Wadt WR. Ab Initio Effective Core Potentials for Molecular Calculations. Potentials for K to Au Including the Outermost Core Orbitals[J]. J. Chem. Phys., 1985, 82(1): 299–310
Couty M, Hall MB. Basis Sets for Transition Metals: Optimized Outer p Functions[J]. J. Comput. Chem., 1996, 17(11): 1 359–1 370
Boys SF, Bernardi F. The Calculation of Small Molecular Interactions by the Differences of Separate Total Energies. Some Procedures with Reduced Errors[J]. Molecular Physics, 1970, 19(4): 553–566
Kar T, Scheiner S. Cooperativity of Conventional and Unconventional Hydrogen Bonds Involving Imidazole[J]. International Journal of Quantum Chemistry, 2006, 106(4): 843–851.
Maksic ZB, Orville-Thomas W J. Pauling’s Legacy: Modern Modelling of the Chemical Bond[M]. Elsevier, Amsterdam, New York, 1999
Li QZ, An XL, Gong BA, et al. Spectroscopic and Theoretical Evidence for the Cooperativity between Red-shift Hydrogen Bond and Blue-shift Hydrogen Bond in DMSO Aqueous Solutions[J]. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2008, 69(1): 211–215
Author information
Authors and Affiliations
Corresponding author
Additional information
Funded by the National Natural Science Foundation of China (No.51278443) and the Shandong Province Natural Science Foundation (ZR2011EEM006)
Rights and permissions
About this article
Cite this article
Liu, Z., Yu, L. & Li, Q. Synergic mechanism of an organic corrosion inhibitor for preventing carbon steel corrosion in chloride solution. J. Wuhan Univ. Technol.-Mat. Sci. Edit. 30, 325–330 (2015). https://doi.org/10.1007/s11595-015-1148-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11595-015-1148-z