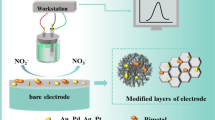
Abstract
Nitrite is widely used due to its personal properties. It not only exists in the food processing and chemical industry but also in every corner of the natural world. Nevertheless, excessive use of nitrite can cause serious environmental and human health hazards. Therefore, the efficient and sensitive detection of nitrite content has become a problem that must be faced. Among numerous methods, the electrochemical method has been widely studied for its advantages, such as fast reaction speed, high sensitivity, and miniaturization. The principle of operation of the nitrite electrochemical sensor is as follows. A modified layer located on the electrode surface oxidizes nitrite to nitrate. The sensing signal generated during oxidation can be converted into a measurable electrochemical signal. The electrochemical signal is proportional to nitrite concentration within a certain range, so that nitrite can be quantitatively detected. However, the sensor’s performance is affected by the high oxidation potential of the bare electrode in electrochemistry. Therefore, the electrode is often modified to improve the sensor’s performance in the study. In this review, the research progress of materials other than precious metals is discussed in the electrochemical detection of nitrite in recent years (2023–2013). The properties of carbon materials, non-noble metals, metal-organic framework compounds, conductive polymers, and their composites in electrochemical sensors are discussed in detail. Besides, it also looks forward to the challenges and prospects of nanomaterials in electrochemical sensor applications.
Graphical Abstract
Article overall summary diagram










Similar content being viewed by others
References
Dai L, Chang DW, Baek JB et al (2012) Carbon nanomaterials for advanced energy conversion and storage. Small 8(8):1130–1166. https://doi.org/10.1002/smll.201101594
Li D, Wang T, Li Z et al (2019) Application of graphene-based materials for detection of nitrate and nitrite in water-a review. Sensors (Basel) 20(1):54. https://doi.org/10.3390/s20010054
Zhang J, Zhang T, Yang J-H (2022) Precious metal nanomaterial-modified electrochemical sensors for nitrite detection. Ionics 28(5):2041–2064. https://doi.org/10.1007/s11581-022-04509-3
Amali RKA, Lim HN, Ibrahim I et al (2021) Significance of nanomaterials in electrochemical sensors for nitrate detection: a review. Trends Environ Anal Chem 31:e00135. https://doi.org/10.1016/j.teac.2021.e00135
Donnelly-Greenan EL, Nevins HM, Harvey JT (2019) Entangled seabird and marine mammal reports from citizen science surveys from coastal California (1997-2017). Mar Pollut Bull 149:110557. https://doi.org/10.1016/j.marpolbul.2019.110557
Li X, Ping J, Ying Y (2019) Recent developments in carbon nanomaterial-enabled electrochemical sensors for nitrite detection. TrAC Trends Anal Chem 113:1–12. https://doi.org/10.1016/j.trac.2019.01.008
Hatta M, Ruzicka JJ, Measures CI (2020) The performance of a new linear light path flow cell is compared with a liquid core waveguide and the linear cell is used for spectrophotometric determination of nitrite in sea water at nanomolar concentrations. Talanta 219:121240. https://doi.org/10.1016/j.talanta.2020.121240
Mao Y, Bao Y, Han D-X et al (2018) Research progress on nitrite electrochemical sensor. Chin J Anal Chem 46(2):147–155. https://doi.org/10.1016/s1872-2040(17)61066-1
Wu J, Wang X, Lin Y et al (2016) Peroxynitrous-acid-induced chemiluminescence detection of nitrite based on microfluidic chip. Talanta 154:73–79. https://doi.org/10.1016/j.talanta.2016.03.062
Kodamatani H, Yamazaki S, Saito K et al (2009) Selective determination method for measurement of nitrite and nitrate in water samples using high-performance liquid chromatography with post-column photochemical reaction and chemiluminescence detection. J Chromatogr A 1216(15):3163–3167. https://doi.org/10.1016/j.chroma.2009.01.096
Chamandust S, Mehrasebi MR, Kamali K et al (2016) Simultaneous determination of nitrite and nitrate in milk samples by ion chromatography method and estimation of dietary intake. Int J Food Prop 19(9):1983–1993. https://doi.org/10.1080/10942912.2015.1091007
Fu Y, Bian C, Kuang J et al (2015) A palladium-tin modified microband electrode array for nitrate determination. Sensors (Basel) 15(9):23249–23261. https://doi.org/10.3390/s150923249
Kalaycıoğlu Z, Erim FB (2015) Simultaneous determination of nitrate and nitrite in fish products with improved sensitivity by sample stacking-capillary electrophoresis. Food Anal Methods 9(3):706–711. https://doi.org/10.1007/s12161-015-0241-4
Xu Z, Shi W, Yang C et al (2020) A colorimetric fluorescent probe for rapid and specific detection of nitrite. Luminescence 35(2):299–304. https://doi.org/10.1002/bio.3727
Bagheri H, Hajian A, Rezaei M et al (2017) Composite of Cu metal nanoparticles-multiwall carbon nanotubes-reduced graphene oxide as a novel and high performance platform of the electrochemical sensor for simultaneous determination of nitrite and nitrate. J Hazard Mater 324(Pt B):762–772. https://doi.org/10.1016/j.jhazmat.2016.11.055
Yang J-H, Yang H, Liu S et al (2015) Microwave-assisted synthesis graphite-supported Pd nanoparticles for detection of nitrite. Sensors Actuators B Chem 220:652–658. https://doi.org/10.1016/j.snb.2015.05.118
Kanoun O, Lazarevic-Pasti T, Pasti I et al (2021) A review of nanocomposite-modified electrochemical sensors for water quality monitoring. Sensors (Basel) 21(12):4131. https://doi.org/10.3390/s21124131
Rassaei L, Marken F, Sillanpää M et al (2011) Nanoparticles in electrochemical sensors for environmental monitoring. TrAC Trends Anal Chem 30(11):1704–1715. https://doi.org/10.1016/j.trac.2011.05.009
Li T, Shang D, Gao S et al (2022) Two-dimensional material-based electrochemical sensors/biosensors for food safety and biomolecular detection. Biosensors (Basel) 12(5):314. https://doi.org/10.3390/bios12050314
Dou B, Yan J, Chen Q et al (2021) Development of an innovative nitrite sensing platform based on the construction of carbon-layer-coated In2O3 porous tubes. Sensors Actuators B Chem 328:129082. https://doi.org/10.1016/j.snb.2020.129082
Li G, Xia Y, Tian Y et al (2019) Review—recent developments on graphene-based electrochemical sensors toward nitrite. J Electrochem Soc 166(12):B881–B895. https://doi.org/10.1149/2.0171912jes
Cheng Z, Song H, Zhang X et al (2022) Enhanced non-enzyme nitrite electrochemical sensing property based on stir bar-shaped ZnO nanorods decorated with nitrogen-doped reduced graphene oxide. Sensors Actuators B Chem 355:131313. https://doi.org/10.1016/j.snb.2021.131313
Lu H, Wang H, Yang L et al (2021) A sensitive electrochemical sensor based on metal cobalt wrapped conducting polymer polypyrrole nanocone arrays for the assay of nitrite. Mikrochim Acta 189(1):26. https://doi.org/10.1007/s00604-021-05131-2
Madasamy T, Pandiaraj M, Balamurugan M et al (2014) Copper, zinc superoxide dismutase and nitrate reductase coimmobilized bienzymatic biosensor for the simultaneous determination of nitrite and nitrate. Biosens Bioelectron 52:209–215. https://doi.org/10.1016/j.bios.2013.08.036
Salagare S, Adarakatti PS, Venkataramanappa Y et al (2021) Electrochemical nitrite sensing employing palladium oxide–reduced graphene oxide (PdO-RGO) nanocomposites: application to food and environmental samples. Ionics 28(2):927–938. https://doi.org/10.1007/s11581-021-04355-9
Salagare S, Adarakatti PS, Yarradoddappa V (2021) Facile synthesis of silver nanoparticle-decorated zinc oxide nanocomposite-based pencil graphite electrode for selective electrochemical determination of nitrite. Carbon Letters 31(6):1273–1286. https://doi.org/10.1007/s42823-021-00251-4
Umapathi R, Venkateswara Raju C, Majid Ghoreishian S et al (2022) Recent advances in the use of graphitic carbon nitride-based composites for the electrochemical detection of hazardous contaminants. Coord Chem Rev 470:214708. https://doi.org/10.1016/j.ccr.2022.214708
Moyo M, Mudarikwa P, Shumba M et al (2017) Voltammetric sensing of nitrite in aqueous solution using titanium dioxide anchored multiwalled carbon nanotubes. Ionics 24(8):2489–2498. https://doi.org/10.1007/s11581-017-2358-5
Liu H-Y, Wen J-J, Huang Z-H et al (2019) Prussian blue analogue of copper-cobalt decorated with multi-walled carbon nanotubes based electrochemical sensor for sensitive determination of nitrite in food samples. Chin J Anal Chem 47(6):e19066–e19072. https://doi.org/10.1016/s1872-2040(19)61168-0
Zhu D, Zhen Q, Xin J et al (2020) A free-standing and flexible phosphorus/nitrogen dual-doped three-dimensional reticular porous carbon frameworks encapsulated cobalt phosphide with superior performance for nitrite detection in drinking water and sausage samples. Sensors Actuators B Chem 321:128541. https://doi.org/10.1016/j.snb.2020.128541
Xian H, Wang P, Zhou Y et al (2010) Electrochemical determination of nitrite via covalent immobilization of a single-walled carbon nanotubes and single stranded deoxyribonucleic acid nanocomposite on a glassy carbon electrode. Microchim Acta 171(1-2):63–69. https://doi.org/10.1007/s00604-010-0404-3
Yang S, Xia B, Zeng X et al (2010) Fabrication of DNA functionalized carbon nanotubes/Cu2+ complex by one-step electrodeposition and its sensitive determination of nitrite. Anal Chim Acta 667(1-2):57–62. https://doi.org/10.1016/j.aca.2010.03.063
Salhi O, Ez-Zine T, Oularbi L et al (2022) Electrochemical sensing of nitrite ions using modified electrode by poly 1,8-diaminonaphthalene/functionalized multi-walled carbon nanotubes. Front Chem 10:870393. https://doi.org/10.3389/fchem.2022.870393
Atta NF, Galal A, Ahmed YM et al (2021) Development of an innovative nitrite sensing platform based on the construction of an electrochemical composite sensor of polymer coated CNTs and decorated with magnetite nanoparticles. Electroanalysis 33(6):1510–1519. https://doi.org/10.1002/elan.202060598
Rashed MA, Faisal M, Alsaiari M et al (2021) MWCNT-doped polypyrrole-carbon black modified glassy carbon electrode for efficient electrochemical sensing of nitrite ions. Electrocatalysis 12(6):650–666. https://doi.org/10.1007/s12678-021-00675-6
Chen Y, Waterhouse GIN, Qiao X et al (2022) Sensitive analytical detection of nitrite using an electrochemical sensor with STAB-functionalized Nb2C@MWCNTs for signal amplification. Food Chem 372:131356. https://doi.org/10.1016/j.foodchem.2021.131356
Lu S, Hummel M, Kang S et al (2020) Selective voltammetric determination of nitrite using cobalt phthalocyanine modified on multiwalled carbon nanotubes. J Electrochem Soc 167(4):046515. https://doi.org/10.1149/1945-7111/ab7982
Mounesh and K. R. Venugopala Reddy (2020) Sensitive and reliable electrochemical detection of nitrite and H2O2 embellish-CoPc coupled with appliance of composite MWCNTs. Anal Chim Acta 1108:98–107. https://doi.org/10.1016/j.aca.2020.02.057
Annalakshmi M, Balasubramanian P, Chen SM et al (2018) Amperometric sensing of nitrite at nanomolar concentrations by using carboxylated multiwalled carbon nanotubes modified with titanium nitride nanoparticles. Mikrochim Acta 186(1):8. https://doi.org/10.1007/s00604-018-3136-4
Rębiś T, Falkowski M, Kryjewski M et al (2019) Single-walled carbon nanotube/sulfanyl porphyrazine hybrids deposited on glassy carbon electrode for sensitive determination of nitrites. Dyes Pigm 171:107660. https://doi.org/10.1016/j.dyepig.2019.107660
Sudha V, Senthil Kumar SM, Thangamuthu R (2018) Simultaneous electrochemical sensing of sulphite and nitrite on acid-functionalized multi-walled carbon nanotubes modified electrodes. J Alloys Compd 749:990–999. https://doi.org/10.1016/j.jallcom.2018.03.287
Arulraj AD, Sundaram E, Vasantha VS et al (2018) Polypyrrole with a functionalized multi-walled carbon nanotube hybrid nanocomposite: a new and efficient nitrite sensor. New J Chem 42(5):3748–3757. https://doi.org/10.1039/c7nj04130f
Lin XR, Zheng YF, Song XC (2018) Fe2O3/MWCNTs nanocomposite decorated glassy carbon electrode for the determination of nitrite. Bull Mater Sci 41(2):35. https://doi.org/10.1007/s12034-018-1553-y
Zhu F, Shi H, Wang C et al (2021) Disposable carbon electrodes modified by a bismuth selenide/carboxylic multiwalled carbon nanotubes composite for the effective electrocatalytic analysis of nitrite. Sensors Actuators B Chem 332:129454. https://doi.org/10.1016/j.snb.2021.129454
Brahem A, Al-Hamry A, Gross MA et al (2022) Stability enhancement of laser-scribed reduced graphene oxide electrodes functionalized by iron oxide/reduced graphene oxide nanocomposites for nitrite sensors. J Compos Sci 6(8):221. https://doi.org/10.3390/jcs6080221
Paisanpisuttisin A, Poonwattanapong P, Rakthabut P et al (2022) Sensitive electrochemical sensor based on nickel/PDDA/reduced graphene oxide modified screen-printed carbon electrode for nitrite detection. RSC Adv 12(45):29491–29502. https://doi.org/10.1039/d2ra03918d
Yuan X, Chen J, Ling Y et al (2022) A facile and efficient nitrite electrochemical sensor based on N, O co-doped porous graphene film. Microchem J 178:107361. https://doi.org/10.1016/j.microc.2022.107361
Sookhakian M, Mat Teridi MA, Tong GB et al (2021) Reduced graphene oxide/copper nanoparticle composites as electrochemical sensor materials for nitrate detection. ACS Appl Nano Mater 4(11):12737–12744. https://doi.org/10.1021/acsanm.1c03351
Suma BP, Pandurangappa M (2019) Graphene oxide/copper terephthalate composite as a sensing platform for nitrite quantification and its application to environmental samples. J Solid State Electrochem 24(1):69–79. https://doi.org/10.1007/s10008-019-04454-8
Tajiki A, Abdouss M, Sadjadi S et al (2020) Voltammetric detection of nitrite anions employing imidazole functionalized reduced graphene oxide as an electrocatalyst. Electroanalysis 32(10):2290–2298. https://doi.org/10.1002/elan.202060187
Madhuvilakku R, Alagar S, Mariappan R et al (2020) Glassy carbon electrodes modified with reduced graphene oxide-MoS2-poly (3, 4-ethylene dioxythiophene) nanocomposites for the non-enzymatic detection of nitrite in water and milk. Anal Chim Acta 1093:93–105. https://doi.org/10.1016/j.aca.2019.09.043
Saranya S, Deepa PN (2020) Evolution of novel rGO/ZrHCF composite and utility in electrocatalysis towards nanomolar detection of sodium nitrite and ferulic acid. J Mater Sci Mater Electron 31(21):18923–18936. https://doi.org/10.1007/s10854-020-04430-3
Rashed MA, Faisal M, Harraz FA et al (2020) rGO/ZnO/Nafion nanocomposite as highly sensitive and selective amperometric sensor for detecting nitrite ions (NO2−). J Taiwan Inst Chem Eng 112:345–356. https://doi.org/10.1016/j.jtice.2020.05.015
Ahammad AJS, Alam MK, Islam T et al (2020) Poly (brilliant cresyl blue)-reduced graphene oxide modified activated GCE for nitrite detection: analyzing the synergistic interactions through experimental and computational study. Electrochim Acta 349:136375. https://doi.org/10.1016/j.electacta.2020.136375
Zhao Z, Zhang J, Wang W et al (2019) Synthesis and electrochemical properties of Co3O4-rGO/CNTs composites towards highly sensitive nitrite detection. Appl Surf Sci 485:274–282. https://doi.org/10.1016/j.apsusc.2019.04.202
Li Y, Cheng C, Yang Y et al (2019) A novel electrochemical sensor based on CuO/H-C3N4/rGO nanocomposite for efficient electrochemical sensing nitrite. J Alloys Compd 798:764–772. https://doi.org/10.1016/j.jallcom.2019.05.137
Rostami M, Abdi G, Kazemi SH et al (2019) Nanocomposite of magnetic nanoparticles/graphene oxide decorated with acetic acid moieties on glassy carbon electrode: a facile method to detect nitrite concentration. J Electroanal Chem 847:113239. https://doi.org/10.1016/j.jelechem.2019.113239
Li L, Liu H, Li B et al (2019) Design and construction of polyaniline/reduced graphene oxide three-dimensional dendritic architecture on interdigital electrode for sensitive detection nitrite. Macromol Res 28(5):455–464. https://doi.org/10.1007/s13233-020-8062-8
Suma BP, Adarakatti PS, Kempahanumakkagari SK et al (2019) A new polyoxometalate/rGO/Pani composite modified electrode for electrochemical sensing of nitrite and its application to food and environmental samples. Mater Chem Phys 229:269–278. https://doi.org/10.1016/j.matchemphys.2019.02.087
Zhang J, Zhang Y, Zhou J et al (2018) Construction of a highly sensitive non-enzymatic nitrite sensor using electrochemically reduced holey graphene. Anal Chim Acta 1043:28–34. https://doi.org/10.1016/j.aca.2018.08.045
Hu J, Zhang J, Zhao Z et al (2017) Synthesis and electrochemical properties of rGO-MoS2 heterostructures for highly sensitive nitrite detection. Ionics 24(2):577–587. https://doi.org/10.1007/s11581-017-2202-y
Wang Y, Cao W, Yin C et al (2018) Nonenzymatic amperometric sensor for nitrite detection based on a nanocomposite consisting of nickel hydroxide and reduced graphene oxide. Electroanalysis 30(12):2916–2924. https://doi.org/10.1002/elan.201800627
Sahoo S, Sahoo PK, Sharma A et al (2020) Interfacial polymerized RGO/MnFe2O4/polyaniline fibrous nanocomposite supported glassy carbon electrode for selective and ultrasensitive detection of nitrite. Sensors Actuators B Chem 309:127763. https://doi.org/10.1016/j.snb.2020.127763
Yue X, Luo X, Zhou Z et al (2019) pH-regulated synthesis of CuOx/ERGO nanohybrids with tunable electrocatalytic oxidation activity towards nitrite sensing. New J Chem 43(12):4947–4958. https://doi.org/10.1039/c9nj00474b
Wang S, Liu M, He S et al (2018) Protonated carbon nitride induced hierarchically ordered Fe2O3/H C3N4/rGO architecture with enhanced electrochemical sensing of nitrite. Sensors Actuators B Chem 260:490–498. https://doi.org/10.1016/j.snb.2018.01.073
Zhang G, Pan P, Yang Z et al (2020) Rapid synthesis of cypress-like CuO nanomaterials and CuO/MWCNTs composites for ultra-high sensitivity electrochemical sensing of nitrite. Microchem J 159:105439. https://doi.org/10.1016/j.microc.2020.105439
Govindasamy M, Wang SF, Huang CH et al (2022) Colloidal synthesis of perovskite-type lanthanum aluminate incorporated graphene oxide composites: electrochemical detection of nitrite in meat extract and drinking water. Microchim Acta 189(5):210. https://doi.org/10.1007/s00604-022-05296-4
Yılmaz-Alhan B, Çelik G, Oguzhan Caglayan M et al (2022) Determination of nitrite on manganese dioxide doped reduced graphene oxide modified glassy carbon by differential pulse voltammetry. Chem Pap 76(8):4919–4925. https://doi.org/10.1007/s11696-022-02218-9
Yue X, Li Y, Li M et al (2021) Three-dimensional porous carbon derived from different organic acid salts for application in electrochemical sensing. RSC Adv 11(50):31834–31844. https://doi.org/10.1039/d1ra05105a
Liu Q, Zhang B, Du S et al (2020) Porous hollow carbon nanospheres as a novel sensing platform for sensitive detection of nitrite in pickle directly. J Appl Electrochem 51(2):295–306. https://doi.org/10.1007/s10800-020-01501-5
Alsaiari M, Saleem A, Alsaiari R et al (2022) SiO2/Al2O3/C grafted 3-n propylpyridinium silsesquioxane chloride-based non-enzymatic electrochemical sensor for determination of carcinogenic nitrite in food products. Food Chem 369:130970. https://doi.org/10.1016/j.foodchem.2021.130970
Dong X, Xie S, Zhu J et al (2021) Mesoporous CoOx/C nanocomposites functionalized electrochemical sensor for rapid and continuous detection of nitrite. Coatings 11(5):596. https://doi.org/10.3390/coatings11050596
Zhang Y, Zhu W, Wang Y et al (2019) High-performance electrochemical nitrite sensing enabled using commercial carbon fiber cloth. Inorg Chem Front 6(6):1501–1506. https://doi.org/10.1039/c9qi00255c
Sivakumar M, Sakthivel M, Chen S-M et al (2017) An electrochemical selective detection of nitrite sensor for polyaniline doped graphene oxide modified electrode. Int J Electrochem Sci 12(6):4835–4846. https://doi.org/10.20964/2017.06.24
Salagare S, Adarakatti PS, Almalki ASA et al (2022) An efficient electrochemical sensor for nitrite based on a mesoporous nickel cobaltite-reduced graphene oxide (NiCo-RGO) nanocomposite. Mater Res Innov 27(4):212–222. https://doi.org/10.1080/14328917.2022.2113678
Gligor D, Walcarius A (2014) Glassy carbon electrode modified with a film of poly(toluidine blue O) and carbon nanotubes for nitrite detection. J Solid State Electrochem 18(6):1519–1528. https://doi.org/10.1007/s10008-013-2365-z
Zhang D, Ma H, Chen Y et al (2013) Amperometric detection of nitrite based on Dawson-type vanodotungstophosphate and carbon nanotubes. Anal Chim Acta 792:35–44. https://doi.org/10.1016/j.aca.2013.07.010
Zhou L, Wang J-P, Gai L et al (2013) An amperometric sensor based on ionic liquid and carbon nanotube modified composite electrode for the determination of nitrite in milk. Sensors Actuators B Chem 181:65–70. https://doi.org/10.1016/j.snb.2013.02.041
Moheimanian N, Raoof JB, Safavi A et al (2013) Nitrite electrochemical sensor for food analysis based on direct immobilization of hemoglobin on multi-walled carbon nanotube ionic liquid electrode. J Iran Chem Soc 11(4):1217–1222. https://doi.org/10.1007/s13738-013-0391-5
Majidi MR, Naseri A, Panahian S et al (2013) Electrocatalytic oxidation and determination of nitrite at multi-walled carbon nanotubes modified carbon ceramic electrode. J Chin Chem Soc 60(3):314–320. https://doi.org/10.1002/jccs.201200365
Mani V, Wu T-Y, Chen S-M (2013) Iron nanoparticles decorated graphene-multiwalled carbon nanotubes nanocomposite-modified glassy carbon electrode for the sensitive determination of nitrite. J Solid State Electrochem 18(4):1015–1023. https://doi.org/10.1007/s10008-013-2349-z
Xu F, Deng M, Liu Y et al (2014) Facile preparation of poly (diallyldimethylammonium chloride) modified reduced graphene oxide for sensitive detection of nitrite. Electrochem Commun 47:33–36. https://doi.org/10.1016/j.elecom.2014.07.016
Liu M, Wang L, Meng Y et al (2014) (4-Ferrocenylethyne) phenylamine functionalized graphene oxide modified electrode for sensitive nitrite sensing. Electrochim Acta 116:504–511. https://doi.org/10.1016/j.electacta.2013.11.060
Gholivand MB, Jalalvand AR, Goicoechea HC (2014) Computer-assisted electrochemical fabrication of a highly selective and sensitive amperometric nitrite sensor based on surface decoration of electrochemically reduced graphene oxide nanosheets with CoNi bimetallic alloy nanoparticles. Mater Sci Eng C 40:109–120. https://doi.org/10.1016/j.msec.2014.03.044
Zhang D, Fang Y, Miao Z et al (2013) Direct electrodeposion of reduced graphene oxide and dendritic copper nanoclusters on glassy carbon electrode for electrochemical detection of nitrite. Electrochim Acta 107:656–663. https://doi.org/10.1016/j.electacta.2013.06.015
Yang YJ, Li W (2014) CTAB functionalized graphene oxide/multiwalled carbon nanotube composite modified electrode for the simultaneous determination of ascorbic acid, dopamine, uric acid and nitrite. Biosens Bioelectron 56:300–306. https://doi.org/10.1016/j.bios.2014.01.037
Wang J, Yin G, Shao Y et al (2008) Electrochemical durability investigation of single-walled and multi-walled carbon nanotubes under potentiostatic conditions. J Power Sources 176(1):128–131. https://doi.org/10.1016/j.jpowsour.2007.10.057
Yang Z, Zhong Y, Zhou X et al (2022) Metal-organic framework-based sensors for nitrite detection: a short review. J Food Meas Charact 16(2):1572–1582. https://doi.org/10.1007/s11694-021-01270-5
Saraf M, Rajak R, Mobin SM (2016) A fascinating multitasking Cu-MOF/rGO hybrid for high performance supercapacitors and highly sensitive and selective electrochemical nitrite sensors. J Mater Chem A 4(42):16432–16445. https://doi.org/10.1039/c6ta06470a
Ping J, Zhou Y, Wu Y et al (2015) Recent advances in aptasensors based on graphene and graphene-like nanomaterials. Biosens Bioelectron 64:373–385. https://doi.org/10.1016/j.bios.2014.08.090
Mehmeti E, Stankovic DM, Hajrizi A et al (2016) The use of graphene nanoribbons as efficient electrochemical sensing material for nitrite determination. Talanta 159:34–39. https://doi.org/10.1016/j.talanta.2016.05.079
Liu Z, Manikandan VS, Chen A (2019) Recent advances in nanomaterial-based electrochemical sensing of nitric oxide and nitrite for biomedical and food research. Curr Opin Electrochem 16:127–133. https://doi.org/10.1016/j.coelec.2019.05.013
Cheng C, Zhang Y, Chen H et al (2023) Reduced graphene oxide-wrapped La(0.8)Sr(0.2)MnO(3) microspheres sensing electrode for highly sensitive nitrite detection. Talanta 260:124644. https://doi.org/10.1016/j.talanta.2023.124644
Magri A, Petriccione M, Gutierrez TJ (2021) Metal-organic frameworks for food applications: a review. Food Chem 354:129533. https://doi.org/10.1016/j.foodchem.2021.129533
Wang C, Liu D, Xie Z et al (2014) Functional metal-organic frameworks via ligand doping: influences of ligand charge and steric demand. Inorg Chem 53(3):1331–1338. https://doi.org/10.1021/ic402015q
Lu W, Wei Z, Gu ZY et al (2014) Tuning the structure and function of metal-organic frameworks via linker design. Chem Soc Rev 43(16):5561–5593. https://doi.org/10.1039/c4cs00003j
Zhang W, Li X, Ding X et al (2023) Progress and opportunities for metal-organic framework composites in electrochemical sensors. RSC Adv 13(16):10800–10817. https://doi.org/10.1039/d3ra00966a
Zou D, Liu D (2019) Understanding the modifications and applications of highly stable porous frameworks via UiO-66. Mater Today Chem 12:139–165. https://doi.org/10.1016/j.mtchem.2018.12.004
Feng L, Hou HB, Zhou H (2020) UiO-66 derivatives and their composite membranes for effective proton conduction. Dalton Trans 49(47):17130–17139. https://doi.org/10.1039/d0dt03051a
Yao M-S, Li W-H, Xu G (2021) Metal–organic frameworks and their derivatives for electrically-transduced gas sensors. Coord Chem Rev 426:213479. https://doi.org/10.1016/j.ccr.2020.213479
Cheng D, Li X, Qiu Y et al (2017) A simple modified electrode based on MIL-53(Fe) for the highly sensitive detection of hydrogen peroxide and nitrite. Anal Methods 9(13):2082–2088. https://doi.org/10.1039/c6ay03164a
Zhao Y, Jiang L, Shangguan L et al (2018) Synthesis of porphyrin-based two-dimensional metal–organic framework nanodisk with small size and few layers. J Mater Chem A 6(6):2828–2833. https://doi.org/10.1039/c7ta07911g
Amali RKA, Lim HN, Ibrahim I et al (2022) A copper-based metal-organic framework decorated with electrodeposited Fe(2)O(3) nanoparticles for electrochemical nitrite sensing. Mikrochim Acta 189(9):356. https://doi.org/10.1007/s00604-022-05450-y
Zhang H-J, Chen W-Y, Zou X et al (2022) A novel copper-functionalized MOF modified composite electrode for high-efficiency detection of nitrite and histamine. J Electrochem Soc 169(7):077511. https://doi.org/10.1149/1945-7111/ac8078
Lu S, Jia H, Hummel M et al (2021) Two-dimensional conductive phthalocyanine-based metal-organic frameworks for electrochemical nitrite sensing. RSC Adv 11(8):4472–4477. https://doi.org/10.1039/d0ra10522h
Arul P, Gowthaman NSK, John SA et al (2020) Ultrasonic assisted synthesis of size-controlled Cu-metal-organic framework decorated graphene oxide composite: sustainable electrocatalyst for the trace-level determination of nitrite in environmental water samples. ACS Omega 5(24):14242–14253. https://doi.org/10.1021/acsomega.9b03829
Suma BP, Pandurangappa M (2020) Hydrothermal synthesis of Zr-amino terephthalate and its composite with MWCNTs as a novel electrode material in nitrite quantification. Electroanalysis 32(11):2493–2502. https://doi.org/10.1002/elan.202060091
Gao F, Tu X, Yu Y et al (2022) Core-shell Cu@C@ZIF-8 composite: a high-performance electrode material for electrochemical sensing of nitrite with high selectivity and sensitivity. Nanotechnology 33(22):225501. https://doi.org/10.1088/1361-6528/ac3da7
Yang Z, Zhou X, Yin Y et al (2022) Metal-organic framework derived rod-like Co@carbon for electrochemical detection of nitrite. J Alloys Compd 911:164915. https://doi.org/10.1016/j.jallcom.2022.164915
Zhang W, Ge C-Y, Jin L et al (2021) Nickel nanoparticles incorporated Co, N co-doped carbon polyhedron derived from core-shell ZIF-8@ZIF-67 for electrochemical sensing of nitrite. J Electroanal Chem 887:115163. https://doi.org/10.1016/j.jelechem.2021.115163
Wang K, Wu C, Wang F et al (2018) In-situ insertion of carbon nanotubes into metal-organic frameworks-derived α-Fe2O3 polyhedrons for highly sensitive electrochemical detection of nitrite. Electrochim Acta 285:128–138. https://doi.org/10.1016/j.electacta.2018.07.228
Dong S, Li Z, Fu Y et al (2020) Bimetal-organic framework Cu-Ni-BTC and its derivative CuO@NiO: construction of three environmental small-molecule electrochemical sensors. J Electroanal Chem 858:113785. https://doi.org/10.1016/j.jelechem.2019.113785
Feng L, Zou M, Lv X et al (2022) Facile synthesis of ZIF-67C@RGO/NiNPs nanocomposite for electrochemical non-enzymatic sensing platform of nitrite. Microchem J 179:107508. https://doi.org/10.1016/j.microc.2022.107508
Zhou X, Zhou Y, Hong Z et al (2018) Magnetic Co@carbon nanocages for facile and binder-free nitrite sensor. J Electroanal Chem 824:45–51. https://doi.org/10.1016/j.jelechem.2018.07.038
Zhe T, Shen S, Li F et al (2023) Bimetallic-MOF-derived crystalline-amorphous interfacial sites for highly efficient nitrite sensing. Food Chem 402:134228. https://doi.org/10.1016/j.foodchem.2022.134228
Arul P, Huang S-T, Mani V et al (2021) Ultrasonic synthesis of bismuth-organic framework intercalated carbon nanofibers: a dual electrocatalyst for trace-level monitoring of nitro hazards. Electrochim Acta 381:138280. https://doi.org/10.1016/j.electacta.2021.138280
Ambaye AD, Muchindu M, Jijana A et al (2023) Screen-printed electrode system based on carbon black/copper-organic framework hybrid nanocomposites for the electrochemical detection of nitrite. Mater Today Commun 35:105567. https://doi.org/10.1016/j.mtcomm.2023.105567
Salagare S, Shivappa Adarakatti P, Venkataramanappa Y (2020) Designing and construction of carboxyl functionalised MWCNTs/Co-MOFs-based electrochemical sensor for the sensitive detection of nitrite. Int J Environ Anal Chem 102(17):5301–5320. https://doi.org/10.1080/03067319.2020.1796989
Sivakumar M, Muthukutty B, Chen T-W et al (2022) Electrocatalytic detection of noxious antioxidant diphenylamine in fruit samples with support of Cu@nanoporous carbon modified sensor. Chemosphere 292:133400. https://doi.org/10.1016/j.chemosphere.2021.133400
Zhao Y, Liu B, Pan L et al (2013) 3D nanostructured conductive polymer hydrogels for high-performance electrochemical devices. Energy Environ Sci 6(10):2856–2870. https://doi.org/10.1039/c3ee40997j
Schiffer L, Shirsath AV, Raël S et al (2022) Electrochemical pressure impedance spectroscopy for polymer electrolyte membrane fuel cells: a combined modeling and experimental analysis. J Electrochem Soc 169(3):034503. https://doi.org/10.1149/1945-7111/ac55cd
Xu M, Zhang H, Zheng J (2022) Polypyrrole microsphere modified porous UiO-66 for electrochemical nitrite sensing. J Electrochem Soc 169(4):047515. https://doi.org/10.1149/1945-7111/ac644c
Li X, Akagi M (2019) Improving multilingual speech emotion recognition by combining acoustic features in a three-layer model. Speech Comm 110:1–12. https://doi.org/10.1016/j.specom.2019.04.004
Mali SM, Narwade SS, Navale YH et al (2019) Facile synthesis of highly porous CuO nanoplates (NPs) for ultrasensitive and highly selective nitrogen dioxide/nitrite sensing. RSC Adv 9(10):5742–5747. https://doi.org/10.1039/c8ra09299k
Manibalan G, Murugadoss G, Hazra S et al (2022) A facile synthesis of Sn-doped CeO2 nanoparticles: high performance electrochemical nitrite sensing application. Inorg Chem Commun 135:109096. https://doi.org/10.1016/j.inoche.2021.109096
Chen L, Zheng J (2022) Two-step hydrothermal and ultrasound-assisted synthesis of CB/NiCo2S4@CeO2 composites for high-sensitivity electrochemical detection of nitrite. Microchem J 181:107717. https://doi.org/10.1016/j.microc.2022.107717
Cheng Z, Song H, Zhang X et al (2022) Non-enzymatic nitrite amperometric sensor fabricated with near-spherical ZnO nanomaterial. Colloids Surf B: Biointerfaces 211:112313. https://doi.org/10.1016/j.colsurfb.2021.112313
Qiu Y, Qu K (2022) Binary organic-inorganic nanocomposite of polyaniline-MnO(2) for non-enzymatic electrochemical detection of environmental pollutant nitrite. Environ Res 214(Pt 3):114066. https://doi.org/10.1016/j.envres.2022.114066
Li Y, Wang T, Wang T et al (2022) Copper oxide nanoleaves covered with loose nickel oxide nanoparticles for sensitive and selective non-enzymatic nitrite sensors. Mater Res Bull 149:111712. https://doi.org/10.1016/j.materresbull.2021.111712
Zhe T, Li M, Li F et al (2022) Integrating electrochemical sensor based on MoO(3)/Co(3)O(4) heterostructure for highly sensitive sensing of nitrite in sausages and water. Food Chem 367:130666. https://doi.org/10.1016/j.foodchem.2021.130666
Shivakumar M, Manjunatha S, Nithyayini KN et al (2021) Electrocatalytic detection of nitrite at NiCo2O4 nanotapes synthesized via microwave-hydrothermal method. J Electroanal Chem 882:115016. https://doi.org/10.1016/j.jelechem.2021.115016
Zhe T, Li R, Wang Q et al (2020) In situ preparation of FeSe nanorods-functionalized carbon cloth for efficient and stable electrochemical detection of nitrite. Sensors Actuators B Chem 321:128452. https://doi.org/10.1016/j.snb.2020.128452
Wang X, Li M, Yang S et al (2020) A novel electrochemical sensor based on TiO2–Ti3C2TX/CTAB/chitosan composite for the detection of nitrite. Electrochim Acta 359:136938. https://doi.org/10.1016/j.electacta.2020.136938
Lu S, Hummel M, Wang X et al (2020) Communication—in situ electrodeposition of nickel phosphide on Ni foam for non-enzymatic detection of nitrite. J Electrochem Soc 167(14):146517. https://doi.org/10.1149/1945-7111/abc99d
Ahammad AJS, Pal PR, Shah SS et al (2019) Activated jute carbon paste screen-printed FTO electrodes for nonenzymatic amperometric determination of nitrite. J Electroanal Chem 832:368–379. https://doi.org/10.1016/j.jelechem.2018.11.034
Dai Y, Huang J, Zhang H et al (2019) Highly sensitive electrochemical analysis of tunnel structured MnO2 nanoparticle-based sensors on the oxidation of nitrite. Sensors Actuators B Chem 281:746–750. https://doi.org/10.1016/j.snb.2018.11.014
Sha R, Gopalakrishnan A, Sreenivasulu KV et al (2019) Template-cum-catalysis free synthesis of α-MnO2 nanorods-hierarchical MoS2 microspheres composite for ultra-sensitive and selective determination of nitrite. J Alloys Compd 794:26–34. https://doi.org/10.1016/j.jallcom.2019.04.251
Vishnu N, Badhulika S (2019) Single step synthesis of MoSe2−MoO3 heterostructure for highly sensitive amperometric detection of nitrite in water samples of industrial areas. Electroanalysis 31(12):2410–2416. https://doi.org/10.1002/elan.201900310
Sun C, Pan W, Zheng D et al (2019) An electrochemical sensor for nitrite using a glassy carbon electrode modified with Cu/CBSA nanoflower networks. Anal Methods 11(39):4998–5006. https://doi.org/10.1039/c9ay01544b
Nithyayini KN, Harish MNK, Nagashree KL (2019) Electrochemical detection of nitrite at NiFe2O4 nanoparticles synthesised by solvent deficient method. Electrochim Acta 317:701–710. https://doi.org/10.1016/j.electacta.2019.06.026
Ma Y, Wang Y, Xie D et al (2018) NiFe-layered double hydroxide nanosheet arrays supported on carbon cloth for highly sensitive detection of nitrite. ACS Appl Mater Interfaces 10(7):6541–6551. https://doi.org/10.1021/acsami.7b16536
Balasubramanian P, Settu R, Chen S-M et al (2018) A new electrochemical sensor for highly sensitive and selective detection of nitrite in food samples based on sonochemical synthesized calcium ferrite (CaFe2O4) clusters modified screen printed carbon electrode. J Colloid Interface Sci 524:417–426. https://doi.org/10.1016/j.jcis.2018.04.036
Sudha V, Mohanty SA, Thangamuthu R (2018) Facile synthesis of Co3O4 disordered circular sheets for selective electrochemical determination of nitrite. New J Chem 42(14):11869–11877. https://doi.org/10.1039/c8nj02639d
Manibalan G, Murugadoss G, Thangamuthu R et al (2018) Enhanced electrochemical supercapacitor and excellent amperometric sensor performance of heterostructure CeO2-CuO nanocomposites via chemical route. Appl Surf Sci 456:104–113. https://doi.org/10.1016/j.apsusc.2018.06.071
Saravanan J, Ramasamy R, Annal Therese H et al (2017) Electrospun CuO/NiO composite nanofibers for sensitive and selective non-enzymatic nitrite sensors. New J Chem 41(23):14766–14771. https://doi.org/10.1039/c7nj02073b
Jaiswal N, Tiwari I, Foster CW et al (2017) Highly sensitive amperometric sensing of nitrite utilizing bulk-modified MnO2 decorated Graphene oxide nanocomposite screen-printed electrodes. Electrochim Acta 227:255–266. https://doi.org/10.1016/j.electacta.2017.01.007
Lu S, Yang C, Nie M (2017) Hydrothermal synthesized urchin-like nickel-cobalt carbonate hollow spheres for sensitive amperometric detection of nitrite. J Alloys Compd 708:780–786. https://doi.org/10.1016/j.jallcom.2017.03.059
Wang H, Chen P, Wen F et al (2015) Flower-like Fe2O3@MoS2 nanocomposite decorated glassy carbon electrode for the determination of nitrite. Sensors Actuators B Chem 220:749–754. https://doi.org/10.1016/j.snb.2015.06.016
Bharath G, Madhu R, Chen S-M et al (2015) Solvent-free mechanochemical synthesis of graphene oxide and Fe3O4–reduced graphene oxide nanocomposites for sensitive detection of nitrite. J Mater Chem A 3(30):15529–15539. https://doi.org/10.1039/c5ta03179f
Huang H, Lv L, Xu F et al (2017) PrFeO3-MoS2 nanosheets for use in enhanced electro-oxidative sensing of nitrite. Microchim Acta 184(10):4141–4149. https://doi.org/10.1007/s00604-017-2446-2
Zhang Y, Chen P, Wen F et al (2016) Fe3O4 nanospheres on MoS2 nanoflake: electrocatalysis and detection of Cr(VI) and nitrite. J Electroanal Chem 761:14–20. https://doi.org/10.1016/j.jelechem.2015.12.004
Wang H, Wen F, Chen Y et al (2016) Electrocatalytic determination of nitrite based on straw cellulose/molybdenum sulfide nanocomposite. Biosens Bioelectron 85:692–697. https://doi.org/10.1016/j.bios.2016.05.078
Zhang Y, Chen P, Wen F et al (2016) Construction of polyaniline/molybdenum sulfide nanocomposite: characterization and its electrocatalytic performance on nitrite. Ionics 22(7):1095–1102. https://doi.org/10.1007/s11581-015-1634-5
Puspalak A, Chinnadurai P, Prathibha R, Kumar MP, Manjushree SG, UdayaKumar V, Adarakatti P (2023) Cobalt oxide nanoparticles based carbon electrode for the detection of residual nitrite in the soil of agricultural fields. Mater Res Innov 27(2):100–109. https://doi.org/10.1080/14328917.2022.2085909
Salagare S, Shivappa Adarakatti P, S. B P et al (2022) A selective electrochemical sensor based on titanium dioxide-reduced graphene oxide nanocomposite (TiO2-RGO/GCE) for the efficient determination of nitrite. Mater Res Innov 27(1):33–44. https://doi.org/10.1080/14328917.2022.2071013
Wang J, Zhao D, Zhang Y et al (2014) A highly sensitive sensor for the detection of nitrite based on a nanoporous Fe2O3–CoO composite. Anal Methods 6(9):3147–3151. https://doi.org/10.1039/c4ay00171k
Rahim A, Santos LSS, Barros SBA et al (2014) Electrochemical detection of nitrite in meat and water samples using a mesoporous carbon ceramic SiO2/C electrode modified with in situ generated manganese(II) phthalocyanine. Electroanalysis 26(3):541–547. https://doi.org/10.1002/elan.201300468
Li Y, Wang H, Liu X et al (2014) Nonenzymatic nitrite sensor based on a titanium dioxide nanoparticles/ionic liquid composite electrode. J Electroanal Chem 719:35–40. https://doi.org/10.1016/j.jelechem.2014.02.006
Verma S, Arya P, Singh A et al (2020) ZnO-rGO nanocomposite based bioelectrode for sensitive and ultrafast detection of dopamine in human serum. Biosens Bioelectron 165:112347. https://doi.org/10.1016/j.bios.2020.112347
Alam MM, Uddin MT, Asiri AM et al (2020) Fabrication of selective l-glutamic acid sensor in electrochemical technique from wet-chemically prepared RuO2 doped ZnO nanoparticles. Mater Chem Phys 251:123029. https://doi.org/10.1016/j.matchemphys.2020.123029
Manjari G, Saran S, Radhakrishanan S et al (2020) Facile green synthesis of Ag–Cu decorated ZnO nanocomposite for effective removal of toxic organic compounds and an efficient detection of nitrite ions. J Environ Manag 262:110282. https://doi.org/10.1016/j.jenvman.2020.110282
Yang W, Bai Y, Li Y et al (2005) Amperometric nitrite sensor based on hemoglobin/colloidal gold nanoparticles immobilized on a glassy carbon electrode by a titania sol-gel film. Anal Bioanal Chem 382(1):44–50. https://doi.org/10.1007/s00216-005-3160-1
Manibalan G, Murugadoss G, Thangamuthu R et al (2020) CeO2-based heterostructure nanocomposite for electrochemical determination of l-cysteine biomolecule. Inorg Chem Commun 113:107793. https://doi.org/10.1016/j.inoche.2020.107793
Khoshroo A, Hosseinzadeh L, Adib K et al (2021) Earlier diagnoses of acute leukemia by a sandwich type of electrochemical aptasensor based on copper sulfide-graphene composite. Anal Chim Acta 1146:1–10. https://doi.org/10.1016/j.aca.2020.12.007
Radhakrishnan S, Krishnamoorthy K, Sekar C et al (2014) A highly sensitive electrochemical sensor for nitrite detection based on Fe2O3 nanoparticles decorated reduced graphene oxide nanosheets. Appl Catal B Environ 148-149:22–28. https://doi.org/10.1016/j.apcatb.2013.10.044
Salhi O, Ez-zine T, El Rhazi M (2021) Hybrid Materials Based on Conducting Polymers for Nitrite Sensing: A Mini Review. Electroanalysis 33(7):1681–1690. https://doi.org/10.1002/elan.202100033
Janaky C, Visy C (2013) Conducting polymer-based hybrid assemblies for electrochemical sensing: a materials science perspective. Anal Bioanal Chem 405(11):3489–3511. https://doi.org/10.1007/s00216-013-6702-y
Ramanavičius A, Ramanavičienė A, Malinauskas A (2006) Electrochemical sensors based on conducting polymer—polypyrrole. Electrochim Acta 51(27):6025–6037. https://doi.org/10.1016/j.electacta.2005.11.052
Tsai M-D, Wang Y-C, Chen Y-L et al (2022) Selectively confined poly(3,4-ethylenedioxythiophene) in the nanopores of a metal–organic framework for electrochemical nitrite detection with reduced limit of detection. ACS Appl Nano Mater 5(9):12980–12990. https://doi.org/10.1021/acsanm.2c02790
Asiri AM, Adeosun WA, Marwani HM et al (2020) Homopolymerization of 3-aminobenzoic acid for enzyme-free electrocatalytic assay of nitrite ions. New J Chem 44(5):2022–2032. https://doi.org/10.1039/c9nj06058h
Islam T, Hasan MM, Akter SS et al (2019) Fabrication of Ni–Co-based heterometallo-supramolecular polymer films and the study of electron transfer kinetics for the nonenzymatic electrochemical detection of nitrite. ACS Appl Polym Mater 2(2):273–284. https://doi.org/10.1021/acsapm.9b00797
Jiao M, Li Z, Li Y et al (2018) Poly(3,4-ethylenedioxythiophene) doped with engineered carbon quantum dots for enhanced amperometric detection of nitrite. Mikrochim Acta 185(5):249. https://doi.org/10.1007/s00604-018-2784-8
Xiao Q, Feng M, Liu Y et al (2017) The graphene/polypyrrole/chitosan-modified glassy carbon electrode for electrochemical nitrite detection. Ionics 24(3):845–859. https://doi.org/10.1007/s11581-017-2247-y
Wang X, Tan W, Ji H et al (2018) Facile electrosynthesis of nickel hexacyanoferrate/poly(2,6-diaminopyridine) hybrids as highly sensitive nitrite sensor. Sensors Actuators B Chem 264:240–248. https://doi.org/10.1016/j.snb.2018.02.171
Shen Y, Zhu G, Yang J et al (2018) Ultrafine copper decorated polypyrrole nanotube electrode for nitrite detection. Ionics 25(1):297–307. https://doi.org/10.1007/s11581-018-2577-4
Qian W, Guo H, Li X et al (2021) An amperometric nitrite sensing platform with enhanced sensitivity based on copper nanoparticle/nanostructured polyaniline hydrogel nanocomposite. Ionics 27(12):5297–5308. https://doi.org/10.1007/s11581-021-04227-2
Li Y, Geng C, Xu X et al (2021) Construction of polythiophene-derivative films as a novel electrochemical sensor for highly sensitive detection of nitrite. Anal Bioanal Chem 413(26):6639–6647. https://doi.org/10.1007/s00216-021-03630-y
Availability of data and materials
This declaration is “not applicable.”
Funding
This study was supported by the National Natural Science Foundation of China (Grant Nos. 21706241, U1404503, 21403053), China Post-doctoral Science Foundation (2020M672305, 2018M642791), and Key Scientific and Technological Project of Henan Province (202102210042).
Author information
Authors and Affiliations
Contributions
Jie Zhang: had the idea for the article, critically revised. Jing-He Yang: had the idea for the article, critically revised. Tingting Zhang: critically revised. Yu-Xi Yang: performed the literature search and data analysis, write a first draft.
Corresponding authors
Ethics declarations
Ethical approval
This declaration is “not applicable.”
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, YX., Zhang, T., Zhang, J. et al. Non-precious metal-modified sensors for nitrite detection. Ionics 29, 3853–3877 (2023). https://doi.org/10.1007/s11581-023-05168-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-023-05168-8