Abstract
Objectives
XR-hysterosalpingography currently represents the gold standard for tubal pathology evaluation. Magnetic resonance-HSG is an innovative technique. With our study, we aim to comprehend if and how MR-HSG, compared to traditional XR-HSG, could give us this additional information in the diagnostic/therapeutic process.
Materials and methods
This study included 19 patients between 30 and 42 years old (average age 37.7) affected by infertility. Patients underwent contextually both XR-HSG and MR-HSG, using a single catheterization. The dynamic MR-HSG exam consisted a MR sequence during contrast administration through the cervical catheter.
Results
Both XR-HSG and MR-HSG documented that 15 of the 19 patients had bilateral tubal patency, while four patients had monolateral tubal patency. However, MR-HSG allowed us to diagnose additional findings:
-
Two active endometriosis foci in adnexal localization and a condition of adenomyosis
-
A unicornuate uterus malformation
-
A submucous uterine myoma near the tubal ostium
-
A decrease of the ovarian reserve in a patient
So MR-HSG could potentially detect in 10/19 (52%) women the cause of their infertility, compared to 4/19 (21%) detected with XR-HSG and about 30% of women would have resulted as false negatives if we only used XR-HSG.
Finally, with a questionnaire, we demonstrated that MR-HSG is less painful than XR-HSG.
Conclusions
These data thus confirm that XR-HSG and MR-HSG present the same diagnostic of assessing tubal patency. We also demonstrated that MR-HSG is able to detect further collateral findings that could likewise be a possible therapeutic target and it could possibly become the new gold standard in female infertility diagnostics.
Similar content being viewed by others
Introduction
Infertility is defined as a couple's inability to conceive after a period of twelve months of regular unprotected intercourse. Infertility globally affects approximately 10–15% of couples [1]. If a couple has already had children but is not able to have more, we talk about secondary infertility. While subfertility happens when there is a fertility index ¾ time lower than normal: this means that some couples may have to wait longer to conceive. Sterility concerns couples that, after a complete diagnostic and therapeutic process, are not able to have pregnancies because of irreversible problems [2]. To assess infertility, we must therefore distinguish female and male causes, not just considering the addition of the two but a complex combination of genetical and environmental factors that may or may not bring to conceiving and to full-term pregnancy.
X-ray hysterosalpingography (XR-HSG) currently represents the gold standard for tubal pathology evaluation, which causes up to 20–30% of female infertility cases. However, this technique exposes the patient to a certain radiation dose [3]. XR-HSG presents a high sensibility (60–98%) but a low specificity (15/80%) in tubal alteration assessment (18%). This low specificity showed considerable differences among studies, probably related to performing and diagnostic interpretation of the exam that is affected by the operator’s ability, by his experience and by the eventual use of drugs such as hyoscine butylbromide, which reduces the spasms induced by the endoluminal contrast injection and prevents some false positives of lacking tubal patency [4,5,6,7,8]. XR-HSG has also a 70–90% rate of success in tubal unblocking and up to 25–30% of pregnancy success in the following two months. However, about 50% of tubes are once more occluded after one year from the procedure [9]. MR hysterosalpingography (MR-HSG) is a new technique that is still not commonly used clinical practice and for which there are just few studies in the literature, with no X-ray exposure, and that is able to investigate collateral findings much better than the traditional technique [10]. Moreover, it causes less pain and discomfort to patients [11]. In our study, we want to examine how high-field MR (3 T) used for MR-HSG to know if could be useful to comprehend female infertility causes.
Materials and methods
The study included 19 patients from October 2019 to February 2020, with age ranging from 30 to 42 years old and an average age of 37.5. The study was prematurely interrupted due to COVID-19 emergency. This pilot study was approved by the Independent Ethics Committee in October 2019. The order in which the two procedures were performed was random. Of these patients, four had already had full-term pregnancies in the past (secondary infertility).
Inclusion criteria
Women older than 18 years old affected by sterility or primary/secondary infertility (unprotected sexual intercourse for about 12 months), who already undergone an ovarian reserve study (hormonal or ultrasound) and the ovulation assessment through ultrasound follicular monitoring, that must be studied with XR-HSG for tubal patency, having already excluded the male factor as the cause of the couple’s sterility or infertility with a seminogram.
Exclusion criteria
Patients who were pregnant or breastfeeding, with pacemaker, metal devices implanted earlier than the 2000 or that were not compatible with 3 T MR, metal splinters in their bodies for accidents, insulin infusion pumps, recent tattoos, unremovable piercings, claustrophobic, with waist circumference bigger than 88 cm, who had adverse reactions to gadolinium or to iodine-based contrast.
In this interventional and prospective study, patients who met the above criteria were selected from the Couple sterility clinic. A detailed description of the procedure was provided and of its linked risks, with signing of a informed consent for both techniques.
Patient preparation
Patients must have done a recent pelvic transvaginal ultrasound (within 6 months), negative vaginal swabs for common and uncommon germs (within 3 months), declare no unprotected sexual intercourse since last menstruation or provide a negative B-HCG test and do a vaginal douching and an evacuative enema the previous evening and the day of the test. Exams are performed between 7 and 12th cycle day. We recommend patients an antibiotic prophylaxis starting the day before the test until complete cover. Just before the exam, a venous access is placed and an antispastic drug is administered: 20 mg/ml of hyoscine butylbromide to reduce pelvic muscle spasms during contrast injection and as an analgesic for the patient.
Patient catheterization
Patient assumes gynaecological position, external genitals are disinfected with antiseptic solution, and then, we dilate the vaginal canal through a speculum, so that we can identify the cervical orifice and proceed with catheterization with a two-way 7F catheter (27.5 cm; Med Italia Biomedica, Modena, Italia), and anchor it with inflatable balloon. In the same day, we performed XR-HSG and MR-HSG with a single catheterization to decrease procedural and infective risks.
Dynamic exam
-
XR-HSG: Inside the X-ray room we acquire a first image with direct digital technique of the patient’s pelvis, to confirm the correct positioning and to exclude radiotransparent or radiopaque collateral findings. We proceed then with another image to evaluate the correct positioning of the catheter and to confirm that the balloon is inflated enough. Once the correct catheterization is confirmed, we proceed with the dynamic examination through fluoroscopic radiography “cine” sequences of 1 image/sec during iodine-based contrast administration through the catheter (Ioversol 350 mgI/ml: between 5 and 10 ml depending on procedural need).
-
MR-HSG: We used a Philips Achieva 3 Tesla Magnetic Resonance (Philips, Andover, MA). The catheterization procedure of MR-HSG is comparable to the one described above, with the difference that a paramagnetic contrast is used (Gadobutrol 1 mmol/ml diluted with NaCl solution with 1:5 ratio; a volume of 5–10 ml was used, depending on procedural need). The protocol we used the following (duration of about 17 min):
-
T2W_TSE sagittal: TE 140 ms; TR 1029 ms; FOV, 260 × 206; matrix, 288 × 192; average, 0.5; slice thickness, 3 mm; AT 35 s. (We did a previous administration of 5 ml of NaCl solution in the endometrial cavity, then evacuated before contrast injection, to better stretch the cavity and a better evaluation of eventual endometrial polyps or submucous myomas in T2-weighted morphological sequences before contrast administration).
-
T2W_TSE axial, using the long axis of the uterus as a reference for a better morphological evaluation of uterus and adnexa, and preliminarily identifying of the course of the tubes;
-
3d_mDIXON T1 axial without contrast, using also fat suppression sequences, for the evaluation of possible hyperintense endometriosis foci: TE, 2 ms; TR, 4 ms; flip angle (FA), 12#; FOV, 320 × 400; matrix, 320 × 224; averages, 2; slice thickness, 3 mm; AT, 30 s;
-
mDIXON_dyn: high speed T1-weighted dynamic sequences during transvaginal contrast administration: TE 1.35 ms, TR 3.6 ms, FOV 230 × 230, matrix, 96 × 95; averages, 0.5; slice thickness, 3 mm; TA, 10 consecutive fases of 5 s, for a total of AT 57,9 s;
-
T1W_TSE_ax spir: TE, 2 ms; TR, 4 ms; FA, 12; FOV, 320 × 400; matrix, 320 × 224; averages, 2; slice thickness, 3 mm; AT, 30 s.
Results
We here present the result regarding tubal patency in the 19 patients of our study (Table 1):
Of the 19 patients treated with XR-HSG and MR-HSG, 15 had a bilateral tubal patency, while four had a monolateral tubal patency.
Our experience allowed us to demonstrate the same diagnostic efficacy for tubal patency of the two techniques, indicating if it were mono- or bilateral (as shown in Fig. 1).
Among the 19 patients, four had unilateral patency and even here MR-HSG confirmed the obstruction in the same side seen in XR-HSG. In these patients, we proceeded with the attempt to unblock the tube through a higher pressure contrast injection, which, however, did not succeed with neither of the two techniques (Figs. 2 and 3. Table 2).
Among the cases with unilateral tubal patency, MR-HSG allowed us to find out that the cause of two of these patients was a condition of bilateral sactosalpinx with no peritoneal spillage of the contrast, especially in the side with higher severity (Fig. 4).
MR-HSG also allowed us to diagnose some collateral findings:
-
A unicornuate uterus malformation (Fig. 5);
-
A submucous uterine myoma near the tubal ostium;
-
Three cases of endometriosis: two adnexal unilateral and one case of non-secretive ademomyosis
-
A decrease of the ovarian reserve in a patient;
The latter two pathologies could not be diagnosed with XR-HSG, and MR resulted essential. None of the above patients were aware of the discovered pathologies. It follows that MR-HSG can give comparable results in the diagnosis of tubal pathology, also providing further information on genital system as the presence of endometriosis o adnexal pathology.
Patients were finally asked to take a questionnaire at the end of the exams in which they were asked to give a numerical value for the perceived pain during both procedures. They were in particular asked to indicate a number from 0 to 10, being 0 no pain and 10 very painful, and we obtained the results in Table 3. These results confirmed a marked reduction in the perceived pain during MR-HSG compared to XR-HSG, probably due to the different contrast density in the two procedures. In the same questionnaire, we also asked if there were any post-procedure complications in the following hours/days, with no report except for a modest discomfort and tenderness in the following hours for which about 40% of the patients used mild analgesics and rest, with complete resolution within 24 h.
Discussion
The first MR-HSG studies were carried out in 1996 on animal models [12] with very good results and hoping for possible and promising outcomes also in humans. The first report on women done by Wiesner et al. in 2001 [13] outlined the first fundamental innovations of MR-HSG compared to the traditional technique, that are the absence of ionizing radiation and the possibility of visualizing the entirety of uterus and ovaries. In the study of Sadowski et al. [14] published in 2008 by the American Journal of Roentgenology, which assessed tubal patency through MR-HSG using 3D time-resolved images of contrast kinetics, there was a higher number of tubal patency identified by MR-HSG rather than XR-HSG. However, this may be due to different catheterization procedures and the use of inflatable balloon in MR-HSG only, as hypnotized by J.E.Silberzweig in the 2009 letter [15], differently from our study where we used the same catheter for both techniques. Therefore, the higher sensibility in tubal patency diagnosis may have been overestimated in the said study and it could explain the comparable results of the two techniques in our analysis. Other studies on MR-HSG follow [10, 16,17,18], that delineate the first protocols, however, they do not compare it with other techniques. In 2019, Volondat et al. [11] confirm the diagnostic accuracy of MR-HSG and the possibility of better diagnosing intra-uterine malformation, as happened in our study with one case of communicant unicornuate uterus (Fig. 5). Volondat’s research also pointed out the better tolerability for the patients of this technique, compared to the traditional one.
These are the procedural risks in order of frequency reported in the literature [16,17,18]:
-
Pain due to catheterization, to contrast injection in the uterine cavity or to the need of higher pressure when trying to unblock the tube (pain can persist in the following hours, so that resting and oral analgesics are recommended)
-
Vasovagal reflex
-
Post-procedural infections
-
Metrorrhagia
-
Allergic reaction to contrast
-
Uterine or tubal perforation
-
Contrast transfer to venous or lymphatic system.
None of the adverse effects above were seen in our study, except for pain. Jagganatan et al. in 2019 [19] confirmed how sensibility, specificity, positive and negative predictive value and diagnostic accuracy in tubal patency were absolutely comparable between the two techniques and that there were no statistically significant differences. This is in accordance with our study because our results are completely overlapping in the assessment of unilateral and bilateral tubal patency. The thing that makes MR-HSG superior to XR-HSG is the ability to consider further fundamental findings to determine the cause of infertility, in particular in our study we found out:
-
(1)
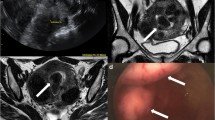
The presence of active endometriosis foci in annexes and adenomyosis (Fig. 6), that may address the clinician towards an effective pharmacological treatment;
-
(2)
The 3D documentation of uterine malformations such as unicornuate uterus (Fig. 5) and a retroversed-anteflexed uterus, badly assessable in XR-HSG;
-
(3)
The presence of a submucous myoma in the tubal ostium that could represent an obstacle for the correct sperm migration and/or of the ovum, the surgical resection of which could resolve the infertility;
-
(4)
The ovarian reserve decrease, which could also be approached form a therapeutic, hormonal or assisted fecundation point of view;
-
(5)
In two patients, we found out that the cause of the lack of tubal patency was high-grade bilateral sactosalpinx, more evident on one side (Fig. 4), for which both techniques gave the same results regarding the tubal patency, however, MR-HSG allowed us to understand the cause behind it.
Since we studied a little number of cases and using descriptive data rather than numerical ones, we can establish that, even with completely comparable results regarding tubal patency, the two techniques have some differences in diagnostic ability for female infertility causes. Among the 15 patients which were negative to the dynamic test, MR-HSG allowed us to diagnose three cases of endometriosis, one submucous myoma, one uterine malformation (communicant unicornuate uterus) and one ovarian reserve decrease. If we consider the sensibility in diagnosing female infertility causes (and not just tubal diseases) we can say that of the 15/19 patients who resulted as negatives with the two techniques, actually 6/15 had another extra-tubal pathology which could possibly be cause or a contributing factor to infertility and that could only be detectable with MR-HSG. So, if we add up these six patients with extra-tubal pathologies with the four patients with known tubal pathology, potentially 10/19 (52%) women could have a cause of infertility detectable with MR-HSG, compared to 4/19 (21%) found with XR-HSG. Our results show that about 30% (52–21%) of women that would have been studied with XR-HSG only, could have resulted as false negatives for infertility determining pathologies (Tables 4 and 5).
Also regarding the therapeutic part of the examination, through the administration of higher pressured contrast in the endocervical catheter to unblock the tube, showed comparable results between the two techniques (Table 2). Both did not succeed, with the only difference that the iodine-based contrast in XR-HSG resulted as more painful for the patients, probably because of its higher density (Table 3).
The growing interest in the last years over MR-HSG and its use [16, 20,21,22], also in comparison with XR-HSG [23], demonstrates its high potential and that it could possibly become the main technique in clinical practice and not only in scientific research. There are also some emerging studies over virtual hysterosalpingography using CT [24], which, however, uses ionizing radiation that, even if in small dose, should be avoided in women of childbearing age, especially if there is a valid and safe alternative such as MR-HSG.
The limits of ISG-MRI consist in the impossibility to subject the patients to such an examination in the presence of absolute contraindications and/or relative to MRI, in particular, the high field (3 T) used in our study and with regard to ISG-RX is the presence of a well-known allergy to non-ionic iodine contrast, statistically more frequent than the allergy to paramagnetic contrast. The limitations of this study are the small number of cases under investigation, partly due to the SARS-Cov2 pandemic.
Conclusions
From our study, we understand that the use of XR-HSG only is not enough to investigate the reproductive system pathology, that will frequently recur to assisted reproductive technology.
In Italy, the low substitution threshold in the population does not allow a generational interchange. The value in 2019 of 1.29 child per woman places our Country among the European states with the lowest levels [25]. This brings a progressive ageing of the population. The average age of our patients was 38 years old, which confirms how often women approach this problem late. Modern society is asking us to adapt to these changes, to the need of women in having a valid aid in conceiving when they decide that their social, financial, working and affective conditions are stable enough to have a child. The woman’s body must be in a perfect balance, in which all components must be suitable to conceiving and to end-term pregnancy. The problems are several and magnetic resonance actually gives the best answer to the problem. In this case more than others, the radiologist has a fundamental part in the diagnostic and therapeutic process, giving both clinician and surgeon an essential collaboration that is necessary for the achieving of the common objective.
Abbreviations
- XR-HSG:
-
Hysterosalpingography with X-ray
- MR-HSG:
-
Hysterosalpingography with magnetic resonance
- MR:
-
Magnetic resonance
References
Tamrakar SR, Bastakoti R (2019) Determinants of infertility in couples. J Nepal Health Res Counc 17(1):85–89. https://doi.org/10.33314/jnhrc.1827
Boivin J, Bunting L, Collins JA, Nygren KG (2007) International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Hum Reprod 22(6):1506–1512. https://doi.org/10.1093/humrep/dem046
Tvarijonaviciene E, Nadisauskiene RJ (2008) The value of hysterosalpingography in the diagnosis of tubal pathology among infertile patients. Medicina (Kaunas) 44(6):439–448
Lim CP, Hasafa Z, Bhattacharya S, Maheshwari A (2011) Should a hysterosalpingogram be a first line investigation to diagnose female tubal subfertility in the modern subfertility workup? Hum Reprod 26(5):967–971
Schankath AC, Fasching N, Urech-Ruh C, Hohl MK, Kubik-Huch RA (2012) Hysterosalpingography in the workup of female infertility: indications, techniques and diagnostic findings. Insights Imaging 3:475–483
Maubon A, Pouquet M, Piver P, Mazet N, Viala-Trentini M, Rouanet JP (2008) Imaging of female infertility. J Radiol 89:172–184
Al Subhi T, Al Jashnmi RN, Al Khaduri M, Gowri V (2013) Prevalence of tubal obstruction in the hysterosalpingogram of women with primary and secondary infertility. J Reprod Infertile 14(4):214–216
Panchal S, Nagori C (2014) Imaging techniques for assessment of tubal status. J Hum Reprod Sci 7(1): 2–12
Passariello R, Simonetti G (2010) Compendio di Radiologia, 3rd edn. Idelson Gnocchi, Italy
Cipolla V, Guerrieri D, Pietrangeli D, Santucci D, Argirò R, De Felice C (2016) Role of 3.0 Tesla magnetic resonance hysterosalpingography in the diagnostic work-up of female infertility. Acta Radiol 57(9):1132–1139
Volondat M, Fontas E, Delotte J et al (2019) Magnetic resonance hysterosalpingography in diagnostic work-up of female infertility—comparison with conventional hysterosalpingography: a randomised study. Eur Radiol 29:501–508
Lee FT Jr, Grist TM, Nelson KG et al (1996) MR hysterosalpingography in a rabbit model. J Magn Reson Imaging 6(2):300–304. https://doi.org/10.1002/jmri.1880060208
Wiesner W, Ruehm SG, Bongartz G, Kaim A, Reese E, De Geyter C (2001) Three-dimensional dynamic MR hysterosalpingography: a preliminary report. Eur Radiol 11(8):1439–1444. https://doi.org/10.1007/s003300000777
Sadowski EA, Ochsner JE, Riherd JM et al (2008) MR hysterosalpingography with an angiographic time-resolved 3D pulse sequence: assessment of tubal patency. AJR 191:1381–1385
Silberzweig JE (2009) MR hysterosalpingography compared with conventional hysterosalpingography. AJR Am J Roentgenol 192(6):W350. https://doi.org/10.2214/AJR.08.2171
Frye RE, Ascher SM, Thomasson D (2000) MR hysterosalpingography: protocol development and refinement for simulating normal and abnormal fallopian tube patency–feasibility study with a phantom. Radiology 214(1):107–112. https://doi.org/10.1148/radiology.214.1.r00ja42107
Winter L, Glücker T, Steimann S et al (2010) Feasibility of dynamic MR-hysterosalpingography for the diagnostic work-up of infertile women. Acta Radiol 51(6):693–701. https://doi.org/10.3109/02841851.2010.482564
Unterweger M, De Geyter C, Fröhlich JM, Bongartz G, Wiesner W (2002) Three-dimensional dynamic MR-hysterosalpingography; a new, low invasive, radiation-free and less painful radiological approach to female infertility. Hum Reprod 17(12):3138–3141. https://doi.org/10.1093/humrep/17.12.3138
Jagannathan D, Hithaya F (2019) Conventional and magnetic resonance hysterosalpingography in assessing tubal patency-a comparative study. Indian J Radiol Imaging 29(2):163–167. https://doi.org/10.4103/ijri.IJRI_109_18
Duan N, Chen X, Yin Y, Wang Z, Chen R (2020) Comparison between magnetic resonance hysterosalpingography and conventional hysterosalpingography: direct visualization of the fallopian tubes using a novel MRI contrast agent mixture. Acta Radiol 61(7):1001–1007. https://doi.org/10.1177/0284185119883712
De Felice C, Rech F, Marini A et al (2012) Magnetic resonance hysterosalpingography in the evaluation of tubal patency in infertile women: an observational study. Clin Exp Obstet Gynecol 39(1):83–88
Ly JQ (2002) Rare bicornuate uterus with fibroid tumors: hysterosalpingography-MR imaging correlation. AJR Am J Roentgenol 179(2):537–538. https://doi.org/10.2214/ajr.179.2.1790537
Chen LS, Zhu ZQ, Li J et al. (2020) Hysterosalpingo-contrast-sonography vs. magnetic resonance-hysterosalpingography for diagnosing fallopian tubal patency: a systematic review and meta-analysis. Eur J Radiol 125: 108891. https://doi.org/10.1016/j.ejrad.2020.108891
Carrascosa PM, Capuñay C, Vallejos J, Martín López EB, Baronio M, Carrascosa JM (2010) Virtual hysterosalpingography: a new multidetector CT technique for evaluating the female reproductive system. Radiographics 30(3): 643–61. https://doi.org/10.1148/rg.303095732
ISTAT (Italian national statistical institute) (2019) Report Indicatori demografici. Istat, Italy. Available via https://www.istat.it/it/files/2020/02/Indicatori-demografici_2019.pdf. Accessed 11 Feb 2020
Acknowledgments
We thank the Policlinico Tor Vergata Foundation and Couple Sterility Clinic of Tor Vergata Hospital.
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Ethical approval
Institutional Review Board approval was obtained.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study. Written informed consent was waived by the Institutional Review Board. (Independent Ethics Committee).
Guarantor
The scientific guarantor of this publication is Dott.ssa Pace Cristina.
Methodology
-
Prospective.
-
Randomized controlled trial.
-
Performed at one institution.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pace, C., Argirò, R., Casadei, L. et al. Comparison between X-ray-hysterosalpingography and 3 Tesla magnetic resonance-hysterosalpingography in the assessment of the tubal patency in the cause of female infertility. Radiol med 127, 1373–1382 (2022). https://doi.org/10.1007/s11547-022-01556-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-022-01556-8