Abstract
Introduction
This study aimed to investigate the relationship between the serum PSA level, Gleason score (GS), PI-RADS v2 score, tumor ADCmin value, and the largest tumor diameter in patients that underwent radical prostatectomy (RP) due to prostate cancer (PCa) and to comparatively evaluate the variables of these parameters in clinically significant and insignificant PCa groups.
Materials and methods
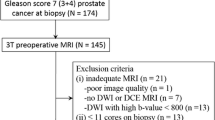
The mpMRI examinations of the patients who underwent RP due to PCa were retrospectively evaluated. According to the final GS, the lesions were divided into two groups as clinically significant (GS ≥ 7) and insignificant (GS ≤ 6). The PSA value, tumor ADCmin value, tumor diameter, and PI-RADS score were compared between the clinically significant and nonsignificant PCa groups using Student’s t-test. The correlations between the serum PSA level, GS, PI-RADS v2 score, tumor ADCmin value, and tumor diameter were evaluated separately (Pearson’s correlation analysis was used for peripheral gland tumors, and Spearman’s correlation analysis for central gland tumors). A ROC analysis was undertaken to evaluate the efficacy of the tumor ADCmin, diameter and PSA values in differentiating clinically significant and nonsignificant tumors.
Results
In both central and peripheral gland tumors, there was a correlation between the PSA level, tumor diameter, PI-RADS score, ADCmin value, and GS at various levels (poor, moderate, and high). In central gland tumors, there was no significant difference between the two groups in terms of the PSA value and PI-RADS scores (p > 0.05), but the ADCmin value and diameter of the tumor significantly differed (p < 0.05). For peripheral gland tumors, significant differences were observed in all parameters (p < 0.05). The cut-off values for the peripheral and central gland tumors are as follows: lesion diameter, 13.5 mm and 19 mm; tumor ADCmin, 0.709 × 10−3 mm2/s and 0.874 × 10−3 mm2/s; and PSA level, 8.47 ng/ml and 11.10 ng/ml, respectively.
Conclusion
The current PI-RADS v2 scoring system can be inadequate in distinguishing clinically significant and insignificant groups in central gland tumors. A separate cut-off value of the tumor diameter should be determined for central and peripheral gland tumors. Tumor ADCmin values can be used as a predictive parameter. The PSA cut-off value should be kept lower in peripheral gland tumors.




Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A (2019) Cancer statistics, 2019. CA Cancer J Clin 69(1):7–34. https://doi.org/10.3322/caac.21551
Johnson LM, Choyke PL, Figg WD, Turkbey B (2014) The role of MRI in prostate cancer active surveillance. Biomed Res Int 2014:203906. https://doi.org/10.1155/2014/203906
Alessandrino F, Taghipour M, Hassanzadeh E, Ziaei A, Vangel M, Fedorov A, Tempany CM, Fennessy FM (2019) Predictive role of PI-RADSv2 and ADC parameters in differentiating Gleason pattern 3 + 4 and 4 + 3 prostate cancer. Abdom Radiol (NY) 44(1):279–285. https://doi.org/10.1007/s00261-018-1718-6
Hassanzadeh E, Glazer DI, Dunne RM, Fennessy FM, Harisinghani MG, Tempany CM (2017) Prostate imaging reporting and data system version 2 (PI-RADS v2): a pictorial review. Abdom Radiol (NY) 42(1):278–289. https://doi.org/10.1007/s00261-016-0871-z
Turkbey B, Brown AM, Sankineni S, Wood BJ, Pinto PA, Choyke PL (2016) Multiparametric prostate magnetic resonance imaging in the evaluation of prostate cancer. CA Cancer J Clin 66(4):326–336. https://doi.org/10.3322/caac.21333
Gupta RT, Spilseth B, Patel N, Brown AF, Yu J (2016) Multiparametric prostate MRI: focus on T2-weighted imaging and role in staging of prostate cancer. Abdom Radiol (NY) 41(5):831–843. https://doi.org/10.1007/s00261-015-0579-5
Starobinets O, Simko JP, Kuchinsky K, Kornak J, Carroll PR, Greene KL, Kurhanewicz J, Noworolski SM (2017) Characterization and stratification of prostate lesions based on comprehensive multiparametric MRI using detailed whole-mount histopathology as a reference standard. NMR Biomed. https://doi.org/10.1002/nbm.3796
Fütterer JJ, Briganti A, De Visschere P, Emberton M, Giannarini G, Kirkham A, Taneja SS, Thoeny H, Villeirs G, Villers A (2015) Can clinically significant prostate cancer be detected with multiparametric magnetic resonance imaging? A systematic review of the literature. Eur Urol 68(6):1045–1053. https://doi.org/10.1016/j.eururo.2015.01.013
Esen T, Turkbey B, Patel A, Futterer J (2014) Multiparametric MRI in prostate cancer. Biomed Res Int 2014:296810. https://doi.org/10.1155/2014/296810
Tamada T, Sone T, Higashi H, Jo Y, Yamamoto A, Kanki A, Ito K (2011) Prostate cancer detection in patients with total serum prostate-specific antigen levels of 4-10 ng/mL: diagnostic efficacy of diffusion-weighted imaging, dynamic contrast-enhanced MRI, and T2-weighted imaging. AJR Am J Roentgenol 197(3):664–670. https://doi.org/10.2214/AJR.10.5923
Kitzing YX, Prando A, Varol C, Karczmar GS, Maclean F, Oto A (2016) Benign conditions that mimic prostate carcinoma: MR imaging features with histopathologic correlation. Radiographics 36(1):162–175. https://doi.org/10.1148/rg.2016150030
Fütterer JJ (2017) Multiparametric MRI in the detection of clinically significant prostate cancer. Korean J Radiol 18(4):597–606. https://doi.org/10.3348/kjr.2017.18.4.597
Borofsky S, George AK, Gaur S, Bernardo M, Greer MD, Mertan FV, Taffel M, Moreno V, Merino MJ, Wood BJ, Pinto PA, Choyke PL, Turkbey B (2018) What are we missing? False-negative cancers at multiparametric MR imaging of the prostate. Radiology 286(1):186–195. https://doi.org/10.1148/radiol.2017152877
Fusco R, Sansone M, Petrillo M, Setola SV, Granata V, Botti G, Perdonà S, Borzillo V, Muto P, Petrillo A (2016) Multiparametric MRI for prostate cancer detection: preliminary results on quantitative analysis of dynamic contrast enhanced imaging, diffusion-weighted imaging and spectroscopy imaging. Magn Reson Imaging 34(7):839–845. https://doi.org/10.1016/j.mri.2016.04.001
Haider MA, Yao X, Loblaw A, Finelli A (2016) Multiparametric magnetic resonance imaging in the diagnosis of prostate cancer: a systematic review. Clin Oncol (R Coll Radiol) 28(9):550–567. https://doi.org/10.1016/j.clon.2016.05.003
Mertan FV, Berman R, Szajek K, Pinto PA, Choyke PL, Turkbey B (2016) Evaluating the role of mpMRI in prostate cancer assessment. Expert Rev Med Devices 13(2):129–141. https://doi.org/10.1586/17434440.2016.1134311
Hoang Dinh A, Melodelima C, Souchon R, Lehaire J, Bratan F, Mège-Lechevallier F, Ruffion A, Crouzet S, Colombel M, Rouvière O (2016) Quantitative analysis of prostate multiparametric MR images for detection of aggressive prostate cancer in the peripheral zone: a multiple imager study. Radiology 280(1):117–127. https://doi.org/10.1148/radiol.2016151406
Ahmed HU, El-Shater Bosaily A, Brown LC, Gabe R, Kaplan R, Parmar MK, Collaco-Moraes Y, Ward K, Hindley RG, Freeman A, Kirkham AP, Oldroyd R, Parker C, Emberton M, PROMIS study group (2017) Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 389(10071):815–822. https://doi.org/10.1016/s0140-6736(16)32401-1
McCann SM, Jiang Y, Fan X, Wang J, Antic T, Prior F, VanderWeele D, Oto A (2016) Quantitative multiparametric MRI features and PTEN expression of peripheral zone prostate cancer: a pilot study. AJR Am J Roentgenol 206(3):559–565. https://doi.org/10.2214/AJR.15.14967
Kasel-Seibert M, Lehmann T, Aschenbach R, Guettler FV, Abubrig M, Grimm MO, Teichgraeber U, Franiel T (2016) Assessment of PI-RADS v2 for the detection of prostate cancer. Eur J Radiol 85(4):726–731. https://doi.org/10.1016/j.ejrad.2016.01.011
Wei C, Jin B, Szewczyk-Bieda M, Gandy S, Lang S, Zhang Y, Huang Z, Nabi G (2018) Quantitative parameters in dynamic contrast-enhanced magnetic resonance imaging for the detection and characterization of prostate cancer. Oncotarget 9(22):15997–16007. https://doi.org/10.18632/oncotarget.24652
Weinreb JC, Barentsz JO, Choyke PL, Cornud F, Haider MA, Macura KJ, Margolis D, Schnall MD, Shtern F, Tempany CM, Thoeny HC, Verma S (2016) PI-RADS prostate imaging—reporting and data system: 2015, version 2. Eur Urol 69(1):16–40. https://doi.org/10.1016/j.eururo.2015.08.052Epub 2015 Oct 1 PubMed PMID: 26427566
Hambrock T, Somford DM, Huisman HJ, van Oort IM, Witjes JA, Hulsbergen-van de Kaa CA, Scheenen T, Barentsz JO (2011) Relationship between apparent diffusion coefficients at 3.0-T MR imaging and Gleason grade in peripheral zone prostate cancer. Radiology 259(2):453–461. https://doi.org/10.1148/radiol.11091409
Oto A, Yang C, Kayhan A, Tretiakova M, Antic T, Schmid-Tannwald C, Eggener S, Karczmar GS, Stadler WM (2011) Diffusion-weighted and dynamic contrast-enhanced MRI of prostate cancer: correlation of quantitative MR parameters with Gleason score and tumor angiogenesis. AJR Am J Roentgenol 197(6):1382–1390. https://doi.org/10.2214/AJR.11.6861
Park JJ, Park BK (2017) Role of PI-RADSv2 with multiparametric MRI in determining who needs active surveillance or definitive treatment according to PRIAS. J Magn Reson Imaging 45(6):1753–1759. https://doi.org/10.1002/jmri.25534
Habibian DJ, Liu CC, Dao A, Kosinski KE, Katz AE (2017) Imaging characteristics of prostate cancer patients who discontinued active surveillance on 3-T multiparametric prostate MRI. AJR Am J Roentgenol 208(3):564–569. https://doi.org/10.2214/AJR.16.16822
Kwak JT, Sankineni S, Xu S, Turkbey B, Choyke PL, Pinto PA, Moreno V, Merino M, Wood BJ (2017) Prostate cancer: a correlative study of multiparametric MR imaging and digital histopathology. Radiology 285(1):147–156. https://doi.org/10.1148/radiol.2017160906
Mottet N, Bellmunt J, Bolla M, Briers E, Cumberbatch MG, De Santis M, Fossati N, Gross T, Henry AM, Joniau S, Lam TB, Mason MD, Matveev VB, Moldovan PC, van den Bergh RCN, Van den Broeck T, van der Poel HG, van der Kwast TH, Rouvière O, Schoots IG, Wiegel T, Cornford P (2017) EAU-ESTRO-SIOG guide-lines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol 71(4):618–629. https://doi.org/10.1016/j.eururo.2016.08.003
An JY, Sidana A, Holzman SA, Baiocco JA, Mehralivand S, Choyke PL, Wood BJ, Turkbey B, Pinto PA (2018) Ruling out clinically significant prostate cancer with negative multi-parametric MRI. Int Urol Nephrol 50(1):7–12. https://doi.org/10.1007/s11255-017-1715-7
Polanec SH, Helbich TH, Bickel H, Wengert GJ, Pinker K, Spick C, Clauser P, Susani M, Shariat S, Baltzer PAT (2018) Quantitative apparent diffusion coefficient derived from diffusion-weighted imaging has the potential to avoid unnecessary MRI-guided biopsies of mpMRI-Detected PI-RADS 4 and 5 lesions. Invest Radiol 53(12):736–741. https://doi.org/10.1097/RLI.0000000000000498
Hauth E, Halbritter D, Jaeger H, Hohmuth H, Beer M (2017) Diagnostic value of semi-quantitative and quantitative analysis of functional parameters in multiparametric MRI of the prostate. Br J Radiol 90(1078):20170067. https://doi.org/10.1259/bjr.20170067
Eldred-Evans D, Neves JB, Simmons LAM, Kanthabalan A, McCartan N, Shah TT, Arya M, Charman SC, Freeman A, Moore CM, Punwani S, Emberton M, Ahmed HU (2019) Added value of diffusion-weighted images and dynamic contrast enhancement in multiparametric magnetic resonance imaging for the detection of clinically significant prostate cancer in the PICTURE trial. BJU Int 11:16. https://doi.org/10.1111/bju.14953
Manetta R, Palumbo P, Gianneramo C, Bruno F, Arrigoni F, Natella R, Maggialetti N, Agostini A, Giovagnoni A, Di Cesare E, Splendiani A, Masciocchi C, Barile A (2019) Correlation between ADC values and Gleason score in evaluation of prostate cancer: multicentre experience and review of the literature. Gland Surg 9(8):216–222. https://doi.org/10.21037/gs.2019.05.02
Gaur S, Harmon S, Rosenblum L, Greer MD, Mehralivand S, Coskun M, Merino MJ, Wood BJ, Shih JH, Pinto PA, Choyke PL, Turkbey B (2018) Can apparent diffusion coefficient values assist PI-RADS version 2 DWI scoring? A correlation study using the PI-RADSv2 and international society of urological pathology systems. AJR Am J Roentgenol 211(1):W33–W41. https://doi.org/10.2214/AJR.17.18702
Turkbey B, Shah VP, Pang Y, Bernardo M, Xu S, Kruecker J, Locklin J, Baccala AA Jr, Rastinehad AR, Merino MJ, Shih JH, Wood BJ, Pinto PA, Choyke PL (2011) Is apparent diffusion coefficient associated with clinical risk scores for prostate cancers that are visible on 3-T MR images? Radiology 258(2):488–495. https://doi.org/10.1148/radiol.10100667
Tamada T, Sone T, Jo Y, Toshimitsu S, Yamashita T, Yamamoto A, Tanimoto D, Ito K (2008) Apparent diffusion coefficient values in peripheral and transition zones of the prostate: comparison between normal and malignant prostatic tissues and correlation with histologic grade. J Magn Reson Imaging 28(3):720–726. https://doi.org/10.1002/jmri.21503
Wang XZ, Wang B, Gao ZQ, Liu JG, Liu ZQ, Niu QL, Sun ZK, Yuan YX (2009) Diffusion-weighted imaging of prostate cancer: correlation between apparent diffusion coefficient values and tumor proliferation. J Magn Reson Imaging 29(6):1360–1366. https://doi.org/10.1002/jmri.21797
Zelhof B, Pickles M, Liney G, Gibbs P, Rodrigues G, Kraus S, Turnbull L (2009) Correlation of diffusion-weighted magnetic resonance data with cellularity in prostate cancer. BJU Int 103(7):883–888. https://doi.org/10.1111/j.1464-410X.2008.08130.x
Jordan EJ, Fiske C, Zagoria R, Westphalen AC (2018) PI-RADS v2 and ADC values: is there room for improvement? Abdom Radiol (NY) 43(11):3109–3116. https://doi.org/10.1007/s00261-018-1557-5
Singh K, Gupta K, Thukral CL, Goyal P, AroraV SI (2018) PI-RADS v2 in prostate cancer and correlation with T staging, PSA levels and ADC values. Iran J Radiol 15(1):e14038. https://doi.org/10.5812/iranjradiol.14038
Hamoen EHJ, de Rooij M, Witjes JA, Barentsz JO, Rovers MM (2015) Use of the prostate imaging reporting and data system (PI-RADS) for prostate cancer detection with multiparametric magnetic resonance imaging: a diagnostic meta-analysis. Eur Urol 67(6):1112–1121. https://doi.org/10.1016/j.eururo.2014.10.033
Sönmez G, Tombul ŞT, Demirtaş T, Öztürk F, Demirtaş A (2019) A comparative study: has MRI-guided fusion prostate biopsy changed the prostate-specific antigen gray-zone range? Cureus 11(12):e6329. https://doi.org/10.7759/cureus.6329
Stojadinovic M, Vukovic I, Ivanovic M, Stojadinovic M, Milovanovic D, Pantic D, Jankovic S (2019) Optimal threshold of the prostate health index in predicting aggressive prostate cancer using predefined cost-benefit ratios and prevalence. Int Urol Nephrol. https://doi.org/10.1007/s11255-019-02367-z
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments os comparable ethical standards.
Informed consent
For this type of study, formal consent is not required. In any case, all persons gave their informed consent to undergo magnetic resonance imaging.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gündoğdu, E., Emekli, E. & Kebapçı, M. Evaluation of relationships between the final Gleason score, PI-RADS v2 score, ADC value, PSA level, and tumor diameter in patients that underwent radical prostatectomy due to prostate cancer. Radiol med 125, 827–837 (2020). https://doi.org/10.1007/s11547-020-01183-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-020-01183-1