Abstract
Purpose
Haralick features Texture analysis is a recent oncologic imaging biomarker used to assess quantitatively the heterogeneity within a tumor. The aim of this study is to evaluate which Haralick’s features are the most feasible in predicting tumor response to neoadjuvant chemoradiotherapy (CRT) in colorectal cancer.
Materials and Methods
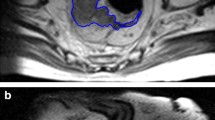
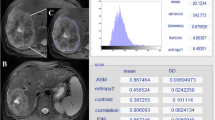
After MRI and histological assessment, eight patients were enrolled and divided into two groups based on response to neoadjuvant CRT in complete responders (CR) and non-responders (NR). Oblique Axial T2-weighted MRI sequences before CRT were analyzed by two radiologists in consensus drawing a ROI around the tumor. 14 over 192 Haralick’s features were extrapolated from normalized gray-level co-occurrence matrix in four different directions. A dedicated statistical analysis was performed to evaluate distribution of the extracted Haralick’s features computing mean and standard deviation.
Results
Pretreatment MRI examination showed significant value (p < 0.05) of 5 over 14 computed Haralick texture. In particular, the significant features are the following: concerning energy, contrast, correlation, entropy and inverse difference moment.
Conclusions
Five Haralick’s features showed significant relevance in the prediction of response to therapy in colorectal cancer and might be used as additional imaging biomarker in the oncologic management of colorectal patients.


Similar content being viewed by others
References
Beets-Tan RG, Lambregts DM, Maas M, Bipat S, Barbaro B, Caseiro-Alves F, Curvo-Semedo L, Fenlon HM, Gollub MJ, Gourtsoyianni S, Halligan S, Hoeffel C, Kim SH, Laghi A, Maier A, Rafaelsen SR, Stoker J, Taylor SA, Torkzad MR, Blomqvist L (2013) Magnetic resonance imaging for the clinical management of rectal cancer patients: recommendations from the 2012 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur Radiol 23(9):2522–2531. https://doi.org/10.1007/s00330-013-2864-4
Laghi A, Ferri M, Catalano C, Baeli I, Iannaccone R, Iafrate F, Ziparo V, Passariello R (2002) Local staging of rectal cancer with MRI using a phased array body coil. Abdom Imaging 27(4):425–431. https://doi.org/10.1107/s00261-001-0123-7
Group MS (2006) Diagnostic accuracy of preoperative magnetic resonance imaging in predicting curative resection of rectal cancer: prospective observational study. BMJ 333(7572):779. https://doi.org/10.1136/bmj.38937.646400.55
van den Broek JJ, van der Wolf FS, Lahaye MJ, Heijnen LA, Meischl C, Heitbrink MA, Schreurs WH (2017) Accuracy of MRI in restaging locally advanced rectal cancer after preoperative chemoradiation. Dis Colon Rectum 60(3):274–283. https://doi.org/10.1097/dcr.0000000000000743
Maas M, Lambregts DM, Lahaye MJ, Beets GL, Backes W, Vliegen RF, Osinga-de Jong M, Wildberger JE, Beets-Tan RG (2012) T-staging of rectal cancer: accuracy of 3.0 Tesla MRI compared with 1.5 Tesla. Abdom Imaging 37(3):475–481. https://doi.org/10.1007/s00261-011-9770-5
Attenberger UI, Pilz LR, Morelli JN, Hausmann D, Doyon F, Hofheinz R, Kienle P, Post S, Michaely HJ, Schoenberg SO, Dinter DJ (2014) Multi-parametric MRI of rectal cancer—do quantitative functional MR measurements correlate with radiologic and pathologic tumor stages? Eur J Radiol 83(7):1036–1043. https://doi.org/10.1016/j.ejrad.2014.03.012
Hotker AM, Tarlinton L, Mazaheri Y, Woo KM, Gonen M, Saltz LB, Goodman KA, Garcia-Aguilar J, Gollub MJ (2016) Multiparametric MRI in the assessment of response of rectal cancer to neoadjuvant chemoradiotherapy: a comparison of morphological, volumetric and functional MRI parameters. Eur Radiol 26(12):4303–4312. https://doi.org/10.1007/s00330-016-4283-9
Curvo-Semedo L, Lambregts DM, Maas M, Beets GL, Caseiro-Alves F, Beets-Tan RG (2012) Diffusion-weighted MRI in rectal cancer: apparent diffusion coefficient as a potential noninvasive marker of tumor aggressiveness. J Magn Reson Imaging JMRI 35(6):1365–1371. https://doi.org/10.1002/jmri.23589
Jung SH, Heo SH, Kim JW, Jeong YY, Shin SS, Soung MG, Kim HR, Kang HK (2012) Predicting response to neoadjuvant chemoradiation therapy in locally advanced rectal cancer: diffusion-weighted 3 Tesla MR imaging. J Magn Reson Imaging JMRI 35(1):110–116. https://doi.org/10.1002/jmri.22749
Cai PQ, Wu YP, An X, Qiu X, Kong LH, Liu GC, Xie CM, Pan ZZ, Wu PH, Ding PR (2014) Simple measurements on diffusion-weighted MR imaging for assessment of complete response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer. Eur Radiol 24(11):2962–2970. https://doi.org/10.1007/s00330-014-3251-5
Intven M, Reerink O, Philippens ME (2015) Dynamic contrast enhanced MR imaging for rectal cancer response assessment after neo-adjuvant chemoradiation. J Magn Reson Imaging JMRI 41(6):1646–1653. https://doi.org/10.1002/jmri.24718
Gollub MJ, Gultekin DH, Akin O, Do RK, Fuqua JL 3rd, Gonen M, Kuk D, Weiser M, Saltz L, Schrag D, Goodman K, Paty P, Guillem J, Nash GM, Temple L, Shia J, Schwartz LH (2012) Dynamic contrast enhanced-MRI for the detection of pathological complete response to neoadjuvant chemotherapy for locally advanced rectal cancer. Eur Radiol 22(4):821–831. https://doi.org/10.1007/s00330-011-2321-1
Hamilton PW, Bartels PH, Thompson D, Anderson NH, Montironi R, Sloan JM (1997) Automated location of dysplastic fields in colorectal histology using image texture analysis. J Pathol 182(1):68–75. https://doi.org/10.1002/(sici)1096-9896(199705)182:1<68:aid-path811>3.0.co;2-n
De Cecco CN, Ganeshan B, Ciolina M, Rengo M, Meinel FG, Musio D, De Felice F, Raffetto N, Tombolini V, Laghi A (2015) Texture analysis as imaging biomarker of tumoral response to neoadjuvant chemoradiotherapy in rectal cancer patients studied with 3-T magnetic resonance. Invest Radiol 50(4):239–245. https://doi.org/10.1097/RLI.0000000000000116
De Cecco CN, Ciolina M, Caruso D, Rengo M, Ganeshan B, Meinel FG, Musio D, De Felice F, Tombolini V, Laghi A (2016) Performance of diffusion-weighted imaging, perfusion imaging, and texture analysis in predicting tumoral response to neoadjuvant chemoradiotherapy in rectal cancer patients studied with 3T MR: initial experience. Abdom Radiol (NY) 41(9):1728–1735. https://doi.org/10.1007/s00261-016-0733-8
Castellano G, Bonilha L, Li LM, Cendes F (2004) Texture analysis of medical images. Clin Radiol 59(12):1061–1069. https://doi.org/10.1016/j.crad.2004.07.008
Ahmed A, Gibbs P, Pickles M, Turnbull L (2013) Texture analysis in assessment and prediction of chemotherapy response in breast cancer. J Magn Reson Imaging JMRI 38(1):89–101. https://doi.org/10.1002/jmri.23971
Haralick RM Shanmugam K, Dinstein I (2007) Textural features for image classification. In: IEEE Xplore Document. IEEE. http://ieeexplore.ieee.org/document/4309314/
Gourtsoyianni S, Doumou G, Prezzi D, Taylor B, Stirling JJ, Taylor NJ, Siddique M, Cook GJR, Glynne-Jones R, Goh V (2017) Primary rectal cancer: repeatability of global and local-regional MR imaging texture features. Radiology. https://doi.org/10.1148/radiol.2017161375
Jalil O, Afaq A, Ganeshan B, Patel UB, Boone D, Endozo R, Groves A, Sizer B, Arulampalam T (2017) Magnetic resonance based texture parameters as potential imaging biomarkers for predicting long-term survival in locally advanced rectal cancer treated by chemoradiotherapy. Colorectal Dis 19(4):349–362. https://doi.org/10.1111/codi.13496
Nketiah G, Elschot M, Kim E, Teruel JR, Scheenen TW, Bathen TF, Selnaes KM (2017) T2-weighted MRI-derived textural features reflect prostate cancer aggressiveness: preliminary results. Eur Radiol 27(7):3050–3059. https://doi.org/10.1007/s00330-016-4663-1
Kaur H, Choi H, You YN, Rauch GM, Jensen CT, Hou P, Chang GJ, Skibber JM, Ernst RD (2012) MR imaging for preoperative evaluation of primary rectal cancer: practical considerations. Radiographics 32(2):389–409. https://doi.org/10.1148/rg.322115122
Freeborough PA, Fox NC (1998) MR image texture analysis applied to the diagnosis and tracking of Alzheimer’s disease. IEEE Trans Med Imaging 17(3):475–479. https://doi.org/10.1109/42.712137
Yoo TS, Ackerman MJ, Lorensen WE, Schroeder W, Chalana V, Aylward S, Metaxas D, Whitaker R (2002) Engineering and algorithm design for an image processing Api: a technical report on ITK–the insight toolkit. Stud Health Technol Inform 85:586–592
Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G (2006) User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage 31(3):1116–1128. https://doi.org/10.1016/j.neuroimage.2006.01.015
Soomro MH, Giunta G, Laghi A, Caruso D, Ciolina M, Marchis CD, Conforto S, Schmid M (2017) Haralick’s texture analysis applied to colorectal T2-weighted MRI: a preliminary study of significance for cancer evolution. In: Biomedical Engineering, 03 Feb 2017. ACTA Press, Alberta https://doi.org/10.2316/P.2016.852-019
Wibmer A, Hricak H, Gondo T, Matsumoto K, Veeraraghavan H, Fehr D, Zheng J, Goldman D, Moskowitz C, Fine SW, Reuter VE, Eastham J, Sala E, Vargas HA (2015) Haralick texture analysis of prostate MRI: utility for differentiating non-cancerous prostate from prostate cancer and differentiating prostate cancers with different Gleason scores. Eur Radiol 25(10):2840–2850. https://doi.org/10.1007/s00330-015-3701-8
Li M, Fu S, Zhu Y, Liu Z, Chen S, Lu L, Liang C (2016) Computed tomography texture analysis to facilitate therapeutic decision making in hepatocellular carcinoma. Oncotarget 7(11):13248–13259. https://doi.org/10.18632/oncotarget.7467
Parmar C, Leijenaar RT, Grossmann P, Rios Velazquez E, Bussink J, Rietveld D, Rietbergen MM, Haibe-Kains B, Lambin P, Aerts HJ (2015) Radiomic feature clusters and prognostic signatures specific for lung and head and neck cancer. Sci Rep 5:11044. https://doi.org/10.1038/srep11044
Incoronato M, Aiello M, Infante T, Cavaliere C, Grimaldi AM, Mirabelli P, Monti S, Salvatore M (2017) Radiogenomic analysis of oncological data: a technical survey. Int J Mol Sci. https://doi.org/10.3390/ijms18040805
Ng F, Ganeshan B, Kozarski R, Miles KA, Goh V (2013) Assessment of primary colorectal cancer heterogeneity by using whole-tumor texture analysis: contrast-enhanced CT texture as a biomarker of 5-year survival. Radiology 266(1):177–184. https://doi.org/10.1148/radiol.12120254
Acknowledgments
This study is funded by AIRC (Associazione Italiana per la Ricerca sul Cancro) Investigator Grant 2013/14129.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The Authors declare that they have no conflict of interest.
Ethical standards
All human and animal studies have been approved by the appropriate ethics committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
All patients gave their informed consent prior to their inclusion in the study.
Rights and permissions
About this article
Cite this article
Caruso, D., Zerunian, M., Ciolina, M. et al. Haralick’s texture features for the prediction of response to therapy in colorectal cancer: a preliminary study. Radiol med 123, 161–167 (2018). https://doi.org/10.1007/s11547-017-0833-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-017-0833-8