Abstract
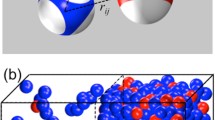
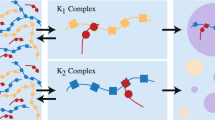
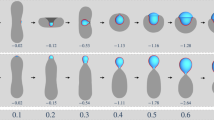
Liquid–liquid phase separation is an emerging mechanism for intracellular organization. This work presents a mathematical model to examine molecular mechanisms that yield phase-separated droplets composed of different RNA–protein complexes. Using a Cahn–Hilliard diffuse interface model with a Flory–Huggins free energy scheme, we explore how multiple (here two, for simplicity) protein–RNA complexes (species) can establish a heterogeneous droplet field where droplets with single or multiple species phase separate and evolve during coarsening. We show that the complex–complex de-mixing energy tunes whether the complexes co-exist or form distinct droplets, while the transient binding kinetics dictate both the timescale of droplet formation and whether distinct species phase separate into droplets simultaneously or sequentially. For specific energetics and kinetics, a field of droplets driven by the formation of only one protein–RNA complex will emerge. Slowly, the other droplet species will accumulate inside the preformed droplets of the other species, allowing them to occupy the same droplet space. Alternatively, unfavorable species mixing creates a parasitic relationship: the slow-to-form protein–RNA complex will accumulate at the surface of a competing droplet species, siphoning off the free protein as it is released. Once this competing protein–RNA complex has sufficiently accumulated on the droplet surface, it can form a new droplet that is capable of sharing an interface with the first complex droplet but is not capable of mixing. These results give insights into a wide range of phase-separation scenarios and heterogeneous droplets that coexist but do not mix within the nucleus and the cytoplasm of cells.







Similar content being viewed by others
Data Availability
All simulation data available on request.
References
Banani SF, Lee HO, Hyman AA, Rosen MK (2017) Biomolecular condensates: organizers of cellular biochemistry. Nat Rev Mol Cell Biol 18(5):285–298
Berry J, Brangwynne CP, Haataja M (2018) Physical principles of intracellular organization via active and passive phase transitions. Rep Prog Phys 81(4):046601
Brangwynne CP, Tompa P, Pappu RV (2015) Polymer physics of intracellular phase transitions. Nat Phys 11(11):899–904
Cahn JW, Hilliard JE (1958) Free energy of a nonuniform system. I. Interfacial free energy. J Chem Phys 28(2):258–267
Chong PA, Vernon RM, Forman-Kay JD (2018) RGG/RG motif regions in RNA binding and phase separation. J Mol Biol 430(23):4650–4665
Dudowicz J, Freed KF, Douglas JF (2004) Flory–Huggins model of equilibrium polymerization and phase separation in the Stockmayer fluid. Phys Rev Lett 92(4):045502
Elbaum-Garfinkle S, Kim Y, Szczepaniak K, Chen CC-H, Eckmann CR, Myong S, Brangwynne CP (2015) The disordered P granule protein LAF-1 drives phase separation into droplets with tunable viscosity and dynamics. Proc Natl Acad Sci 112(23):7189–7194
Feric M, Vaidya N, Harmon TS, Mitrea DM, Zhu L, Richardson TM, Kriwacki RW, Pappu RV, Brangwynne CP (2016) Coexisting liquid phases underlie nucleolar subcompartments. Cell 165(7):1686–1697
Gasior K, Zhao J, McLaughlin G, Forest MG, Gladfelter AS, Newby J (2019) Partial demixing of RNA-protein complexes leads to intradroplet patterning in phase-separated biological condensates. Phys Rev E 99(1):012411
Gladfelter AS (2006) Nuclear anarchy: asynchronous mitosis in multinucleated fungal hyphae. Curr Opin Microbiol 9(6):547–552
Glotzer SC, Di Marzio EA, Muthukumar M (1995) Reaction-controlled morphology of phase-separating mixtures. Phys Rev Lett 74(11):2034
Hult C, Adalsteinsson D, Vasquez PA, Lawrimore J, Bennett M, York A, Cook D, Yeh E, Forest MG, Bloom K (2017) Enrichment of dynamic chromosomal crosslinks drive phase separation of the nucleolus. Nucleic Acids Res 45(19):11159–11173
Hyman AA, Weber CA, Jülicher F (2014) Liquid-liquid phase separation in biology. Annu Rev Cell Dev Biol 30:39–58
Langdon EM, Gladfelter AS (2018) A new lens for RNA localization: liquid-liquid phase separation. Annu Rev Microbiol 72:255–271
Langdon EM, Qiu Y, Niaki AG, McLaughlin GA, Weidmann C, Gerbich TM, Smith JA, Crutchley JM, Termini CM, Weeks KM (2018) mRNA structure determines specificity of a polyQ-driven phase separation. Science 360:922–927
Lee C, Zhang H, Baker AE, Occhipinti P, Borsuk ME, Gladfelter AS (2013a) Protein aggregation behavior regulates cyclin transcript localization and cell-cycle control. Dev Cell 25(6):572–584
Lee CF, Brangwynne CP, Gharakhani J, Hyman AA, Jülicher F (2013b) Spatial organization of the cell cytoplasm by position-dependent phase separation. Phys Rev Lett 111(8):088101
Lee C, Occhipinti P, Gladfelter AS (2015) PolyQ-dependent RNA-protein assemblies control symmetry breaking. J Cell Biol 208:533–544
Lin Y, Protter DS, Rosen MK, Parker R (2015) Formation and maturation of phase-separated liquid droplets by RNA-binding proteins. Mol Cell 60(2):208–219
Molliex A, Temirov J, Lee J, Coughlin M, Kanagaraj AP, Kim HJ, Mittag T, Taylor JP (2015) Phase separation by low complexity domains promotes stress granule assembly and drives pathological fibrillization. Cell 163(1):123–133
Pak CW, Kosno M, Holehouse AS, Padrick SB, Mittal A, Ali R, Yunus AA, Liu DR, Pappu RV, Rosen MK (2016) Sequence determinants of intracellular phase separation by complex coacervation of a disordered protein. Mol Cell 63(1):72–85
Qin R, Bhadeshia H (2010) Phase field method. Mater Sci Technol 26(7):803–811
Schaefer MH, Wanker EE, Andrade-Navarro MA (2012) Evolution and function of CAG/polyglutamine repeats in protein–protein interaction networks. Nucleic Acids Res 40(10):4273–4287
Shen J, Yang X (2010) Numerical approximations of allen-cahn and cahn-hilliard equations. Discrete Contin Dyn Syst 28(4):1669–1691
Tetz G, Tetz V (2017) Prion-like domains in phagobiota. Front Microbiol 8:2239
Walker B, Taylor D, Lawrimore J, Hult C, Adalsteinsson D, Bloom K, Forest MG (2019) Transient crosslinking kinetics optimize gene cluster interactions. PLoS Comput Biol 15(8):e1007124
Weber CA, Zwicker D, Jülicher F, Lee CF (2019) Physics of active emulsions. Rep Prog Phys 82(6):064601
Yang X, Zhao J, He X (2018) Linear, second order and unconditionally energy stable schemes for the viscous Cahn–Hilliard equation with hyperbolic relaxation using the invariant energy quadratization method. J Comput Appl Math 343:80–97
Zhang H, Elbaum-Garfinkle S, Langdon EM, Taylor N, Occhipinti P, Bridges AA, Brangwynne CP, Gladfelter AS (2015) RNA controls PolyQ protein phase transitions. Mol Cell 60(2):220–230
Zwicker D, Seyboldt R, Weber CA, Hyman AA, Jülicher F (2017) Growth and division of active droplets provides a model for protocells. Nat Phys 13(4):408
Acknowledgements
Kelsey Gasior was supported in part by NSF DMS-1816630. M. Gregory Forest was supported in part by NSF DMS-1816630, DMS-1517274, and DMS-1664645. Amy Gladfelter was supported in part by NIH GM R01-GM081506. Jay M. Newby was supported by the Natural Sciences and Engineering Research Council of Canada (RGPIN-2019-06435, RGPAS-2019-00014, DGECR-2019-00321) and the NSF (DMS-171474, DMS-1816630).
Funding
Kelsey Gasior was supported in part by NSF DMS-1816630. M. Gregory Forest was supported in part by NSF DMS-1816630, DMS-1517274, and DMS-1664645. Amy Gladfelter was supported in part by NIH GM R01-GM081506. Jay M. Newby was supported by the Natural Sciences and Engineering Research Council of Canada (RGPIN-2019-06435, RGPAS-2019-00014, DGECR-2019-00321) and the NSF (DMS-171474, DMS-1816630).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Code Availability
Code was created using Matlab.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gasior, K., Forest, M.G., Gladfelter, A.S. et al. Modeling the Mechanisms by Which Coexisting Biomolecular RNA–Protein Condensates Form. Bull Math Biol 82, 153 (2020). https://doi.org/10.1007/s11538-020-00823-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11538-020-00823-x