Abstract
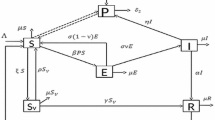
A new two-strain model, for assessing the impact of basic control measures, treatment and dose-structured mass vaccination on cholera transmission dynamics in a population, is designed. The model has a globally-asymptotically stable disease-free equilibrium whenever its associated reproduction number is less than unity. The model has a unique, and locally-asymptotically stable, endemic equilibrium when the threshold quantity exceeds unity and another condition holds. Numerical simulations of the model show that, with the expected 50 % minimum efficacy of the first vaccine dose, vaccinating 55 % of the susceptible population with the first vaccine dose will be sufficient to effectively control the spread of cholera in the community. Such effective control can also be achieved if 50 % of the first vaccine dose recipients take the second dose. It is shown that a control strategy that emphasizes the use of antibiotic treatment is more effective than one that emphasizes the use of basic (non-pharmaceutical) anti-cholera control measures only. Numerical simulations show that, while the universal strategy (involving all three control measures) gives the best outcome in minimizing cholera burden in the community, the combined basic anti-cholera control measures and treatment strategy also has very effective community-wide impact.









Similar content being viewed by others
References
Alexanderian, A., Gobbert, M., Fister, K., Gaff, H., Lenhart, S., & Schaefer, E. (2011). An age-structured model for the spread of epidemic cholera: analysis and simulation. Nonlinear Anal., Real World Appl., 12, 3483–3498.
Anderson, R. M., & May, R. M. (1982). Population biology of infectious diseases. Berlin: Springer.
Blower, S. M., & Dowlatabadi, H. (1994). Sensitivity and uncertainty analysis of complex models of disease transmission: an HIV model, as an example. Int. Stat. Rev., 62, 229–243.
Capasso, V., & Paveri-Fontana, S. L. (1979). A mathematical model for the 1973 cholera epidemic in the European Mediterranean region. Rev. épidémiol. Santé Publique, 27, 121–132.
Carr, J. (1981). Applications of centre manifold theory. New York: Springer.
Castillo-Chavez, C., & Song, B. (2004). Dynamical models of tuberculosis and their applications. Math. Biosci. Eng., 1, 361–404.
Clemens, J. D., Sack, D. A., Harris, J. R., van Loon, F., et al. (1990). Field trial of oral cholera vaccines in Bangladesh: results from three-year follow-up. Lancet, 335, 270–273.
Codeco, C. T. (2001). Endemic and epidemic dynamics of cholera: the role of the aquatic reservoir. BMC Infect. Dis. doi:10.1186/1471-2334-1-1.
Colwell, R. R., & Huq, A. (1994). Environmental reservoir of Vibrio cholerae, the causative agent of cholera. Ann. N.Y. Acad. Sci., 740, 44–53.
Diekmann, O., Heesterbeek, J. A. P., & Metz, J. A. J. (1990). On the definition and computation of the basic reproduction ratio \(\mathcal{R}_{0}\) in models for infectious disease in heterogeneous population. J. Math. Biol., 28, 365–382.
Gaffga, N. H., Tauxe, R. V., & Mintz, E. D. (2007). Cholera: a new home land in Africa? Am. J. Trop. Med. Hyg., 77, 705–713.
Ghosh, A., & Ramamurthy, T. (2011). Antimicrobials and cholera: are we stranded? Indian J. Med. Res., 133, 225–231.
Hale, J. K. (1969). Ordinary differential equations. New York: Wiley.
Hartley, D. M., Morris, J. G. Jr., & Smith, D. L. (2006). Hiperinfectivity: a critical element in the ability of V. cholerae to cause epidemics? PLoS Med., 3(1), 63–69.
Hethcote, H. W. (2000). The mathematics of infectious diseases. SIAM Rev., 42, 599–653.
Hill, D. R., Ford, L., & Lalloo, D. G. (2006). Oral cholera vaccines: use in clinical practice. Lancet Infect. Dis., 7, 361–373.
Hove-Musekwa, S. D., Nyabadza, F., Chiyaka, C., Das, P., Tripathi, A., & Mukandavire, Z. (2011). Modelling and analysis of the effects of malnutrition in the spread of cholera. Math. Comput. Model., 53, 1583–1595.
Islam, M., Miah, M., Hasan, M., Sack, R., & Albert, M. (1994). Detection of non-culturableVibrio cholerae O1 associated with a cyanobacterium from an aquatic environment in Bangladesh. Trans. R. Soc. Trop. Med. Hyg., 88, 298–299.
Kitaoka, M., Miyata, S. T., Unterweger, D., & Pukatzki, S. (2011). Antibiotic resistance mechanisms of Vibrio cholera. J. Med. Microbiol., 60, 397–407.
Longini, I. M. Jr., Nizam, A., Ali, M., Yunus, M., Shenvi, N., & Clemens, J. D. (2007). Controlling endemic cholera with oral vaccines. PLoS Med., 4(11), 1776–1783.
McLeod, R. G., Brewster, J. F., Gumel, A. B., & Slonowsky, D. A. (2006). Sensitivity and uncertainty analyses for a SARS model with time-varying inputs and outputs. Math. Biosci. Eng., 3(3), 527–544.
Mahalanabis, A., Lopez, A. L., Sur, D., Deen, J., Manna, B., et al. (2008). A randomized, placebo-controlled trial of the bivalent killed, whole-cell, oral cholera vaccine in adults and children in a cholera endemic area in Kolkata, India. PLoS ONE, 3(6), 1–7.
Mandal, J., Sangeetha, V., Ganesan, V., Parveen, M., Preethi, V., Harish, B. N., Srinivasan, S., Parija, S. C., & Srinivasan, S. P. (2012). Third-generation cephalosporin-resistant Vibrio cholerae, India. Emerg. Infect. Dis. doi:10.3201/eid1808.111686.
Pascual, M., Bouma, M. J., & Dobson, A. P. (2002). Cholera and climate: revisiting the quantitative evidence. Microbes Infect., 4(2), 237–245.
Sack, D. A. (2011). How many cholera deaths can be averted in Haiti? Lancet, 377, 1214–1216.
Safi, M. A., & Gumel, A. B. (2010). Global asymptotic dynamics of a model for quarantine and isolation. Discrete Contin. Dyn. Syst., Ser. B, 14, 209–231.
Sanches, R. P., Ferreira, C. P., & Kraenkel, R. A. (2011). The role of immunity and seasonality in cholera epidemics. Bull. Math. Biol., 73, 2916–2931.
Seidlein, L. V. (2007). Vaccines for cholera control: does herd immunity play a role? PLoS Med., 4(11), 1719–1721.
Sepulveda, J., Gomez-Dantes, H., & Bronfman, M. (1992). Cholera in the Americas: an overview. Infection, 20, 243–248.
Sharomi, O., & Gumel, A. (2008). Dynamical analysis of a multi-strain model of HIV in the presence of anti-retroviral drugs. J. Biol. Dyn., 2, 323–345.
Shuai, Z., & van den Driessche, P. (2011). Global dynamics of cholera models with differential infectivity. Math. Biosci., 234, 118–126.
Smith, H. L., & Waltman, P. (1995). The theory of the chemostat. Cambridge: Cambridge University Press.
Sur, D., et al. (2009). Efficacy and safety of a modified killed-whole-cell oral cholera vaccine in India: an interim analysis of a cluster-randomised, double-blind, placebo-controlled trial. Lancet, 349, 1694–1702.
Tian, J., & Wang, J. (2011). Global stability for cholera epidemic models. Math. Biosci., 232, 31–41.
Thieme, H. R. (2003). Mathematics in population biology. Princeton: Princeton University Press.
van den Driessche, P., & Watmough, J. (2002). Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Math. Biosci., 180, 29–48.
van Loon, F. P. L., Clemens, D., Chakraborty, J., et al. (1996). Field trial of inactivated cholera vaccines in Bangladesh: results from 5 years of follow-up. Vaccine, 14, 162–166.
World Health Organization (2001). Antimicrobial resistance in shigellosis, cholera and campylobacteriosis. http://www.who.int/drugresistance/Antimicrobial_resistance_in_shigellosis_cholera_and_cam.pdf. Accessed: 10 February 2013.
World Health Organization (2010a). High hopes for oral cholera vaccine. Bull. World Health Organ., 88(3), 165–166.
World Health Organzation (2010b). Cholera vaccines: a brief summary of the March 2010 position paper. http://www.who.int/immunization/Cholera_PP_Accomp_letter__Mar_10_2010.pdf. Accessed: 10 February 2013.
World Health Organzation (2012). Weekly epidemiological, record No. 31-32. http://www.who.int/wer/2012/wer8731_32.pdf. Accessed: 10 February 2013.
World Health Organization (2013). Media center: cholera. http://www.who.int/mediacentre/factsheets/fs107/en/. Accessed: 10 February 2013.
Zhou, X., & Cui, J. (2010). Modeling and stability analysis for a cholera model with vaccination. Math. Methods Appl. Sci., 34, 1711–1724.
Zuckerman, J. N., Rombo, L., & Fisch, A. (2007). The true burden and risk of cholera: implications for prevention and control. Lancet Infect. Dis., 7(8), 521–530.
Acknowledgements
ABG acknowledges, with thanks, the support in part of the Natural Science and Engineering Research Council (NSERC) of Canada. DYM acknowledges the support from the Centre for Global Public Health at the University of Manitoba. The authors are grateful to the anonymous reviewers for their constructive comments.
Author information
Authors and Affiliations
Corresponding author
Appendix: Proof of Theorem 4
Appendix: Proof of Theorem 4
Proof
Let \(\mathcal{R}_{\mathrm{vac}}>1\) and b 1 b 2>0 (so that the model (1) has a unique EEP, in line with Theorem 3). It is convenient to make the following change of variables:

Furthermore, let X=(x 1,x 2,x 3,x 4,x 5,x 6,x 7,x 8,x 9,x 10,x 11)T. Thus, the model (1) can be re-written in the form \(\frac{dX}{dt} = F(X)\), with F=(f 1,f 2,f 3,f 4,f 5,f 6,f 7,f 8,f 9,f 10,f 11)T, as follows:

The Jacobian of the system (19), at the associated DFE (\(\mathcal{E}_{0}\)), is given by
where
Consider the case when \(\mathcal{R}_{\mathrm{vac}}=1\). Furthermore, suppose that β is chosen as a bifurcation parameter. Solving for β from \(\mathcal{R}_{\mathrm{vac}}=1\) gives

with

The transformed system (19), with β=β ∗, has a hyperbolic equilibrium point (i.e., the linearized system has a simple eigenvalue with zero real part, and all other eigenvalues have negative real part), so that the center manifold theory (Carr 1981; Castillo-Chavez and Song 2004) can be used to analyze the dynamics of (19) near β=β ∗.
It can be shown that the right eigenvector of \(J(\mathcal{E}_{0})|_{\beta=\beta^{*}}\), denoted by \({\bf w}\), is given by w=(w 1,w 2,…,w 10,w 11)T, with

It should be noted that w 4,w 5,…,w 10 are positive since they can easily be written in terms of w 11 (and not reported here.) Similarly, \(J(\mathcal{E}_{0})|_{\beta=\beta^{*}}\) has a left eigenvector, \({\bf v}\), given by v=(v 1,v 2,…,v 10,v 11), with

Consequently, it follows that the associated bifurcation coefficients, a and b (defined in Theorem 4.1 of Castillo-Chavez and Song 2004), are given, respectively, by


By substituting (20) in (21), and simplifying, it can be shown that the bifurcation coefficient, a, is negative. The proof is concluded using Part (iv) of Theorem 4.1 in Castillo-Chavez and Song (2004). □
Rights and permissions
About this article
Cite this article
Safi, M.A., Melesse, D.Y. & Gumel, A.B. Dynamics Analysis of a Multi-strain Cholera Model with an Imperfect Vaccine. Bull Math Biol 75, 1104–1137 (2013). https://doi.org/10.1007/s11538-013-9845-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-013-9845-2