Abstract
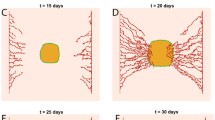
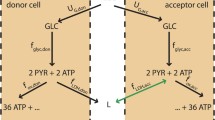
We propose a mathematical model of tumor cell nutrient uptake governed by the presence of two key biomolecular fuels: glucose and lactate. The model allows us to describe, in a remarkably simple way, different in vitro scenarios previously reported in experiments of tumor cell metabolism using distinct energy sources. The predictions of our model show good agreement with all the examined tumor cell lines (cervix, colon, and glioma) and provide a first step toward the development of more comprehensive frameworks accounting for in vivo cancer dynamics under complex spatial heterogeneities.




Similar content being viewed by others
References
Baumann, F., Leukel, P., Doerfelt, A., Beier, C. P., Dettmer, K., Oefner, P. J., Kastenberger, M., Kreutz, M., Nickl-Jockschat, T., Bogdahn, U., Bosserhoff, A.-K., & Hau, P. (2009). Lactate promotes glioma migration by TGF-β2 dependent regulation of matrix metalloproteinase-2. Neuro-oncology, 11(4), 368–380.
Bertuzzi, A., Fasano, A., Gandolfi, A., & Sinisgalli, C. (2010). Necrotic core in EMT6/Ro tumour spheroids: Is it caused by an ATP deficit? J. Theor. Biol., 262, 142–150.
Bonnet, S., Archer, S. L., Allalunis-Turner, J., Haromy, A., Beaulieu, C., Thompson, R., Lee, C. T., Lopaschuk, G. D., Puttagunta, L., Bonnet, S., Harry, G., Hashimoto, K., Porter, C. J., Andrade, M. A., Thebaud, B., & Michelakis, E. D. (2007). A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell, 11, 37–51.
Bonuccelli, G., Tsirigos, A., Whitaker-Menezes, D., Pavlides, S., Pestell, R. G., Chiavarina, B., Frank, P. G., Flomenberg, N., Howell, A., Martinez-Outschoorn, U. E., Sotgia, F., & Lisanti, M. P. (2010). Ketones and lactate “fuel” tumor growth and metastasis. Evidence that epithelial cancer cells use oxidative mitochondrial metabolism. Cell Cycle, 9, 3514–3596.
Bouzier, A.-K., Goodwin, R., de Gannes, F. M., Valeins, H., Voisin, P., Canioni, P., & Merle, M. (1998). Compartmentation of lactate and glucose metabolism in C6 glioma cells. A 13c and 1H NMR study. J. Biol. Chem., 273, 27162–27169.
Bouzier-Sore, A. K., Canioni, P., & Merle, M. (2001). Effect of exogenous lactate on rat glioma metabolism. J. Neurosci. Res., 65, 543–548.
Brahimi-Horn, M. C., Chiche, J., & Pouyssegur, J. (2007). Hypoxia signalling controls metabolic demand. Curr. Opin. Cell Biol., 19, 223–229.
Bristow, R. G., & Hill, R. P. (2008). Hypoxia and metabolism: Hypoxia, DNA repair and genetic instability. Nat. Rev., 8, 180–192.
Buckingham, S. C., Campbell, S. L., Haas, B. R., Montana, V., Robel, S., Ogunrinu, T., & Sontheimer, H. (2011). Glutamate release by primary brain tumors induces epileptic activity. Nat. Med., 17, 1269–1274.
Cairns, R. A., Harris, I. S., & Mak, T. W. (2011). Regulation of cancer cell metabolism. Nat. Rev., Cancer, 11, 85–95.
Chiche, J., Brahimi-Horn, M. C., & Pouysségur, J. (2010). Tumour hypoxia induces a metabolic shift causing acidosis: a common feature in cancer. J. Cell. Mol. Med., 14, 771–794.
De Berardinis, R. J., Mancuso, A., Daikhin, E., Nissim, I., Yudkoff, M., Wehrli, S., & Thompson, C. B. (2007). Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc. Natl. Acad. Sci. USA, 104, 19345–19350.
Elstrom, R. L., Bauer, D. E., Buzzai, M., Karnauskas, R., Harris, M. H., Plas, D. R., Zhuang, H., Cinalli, R. M., Alavi, A., Rudin, C. M., & Thompson, C. B. (2004). Akt stimulates aerobic glycolysis in cancer cells. Cancer Res., 64, 3892–3899.
Fang, J., Quinones, Q. J., Holman, T. L., Morowitz, M. J., Wang, Q., Zhao, H., Sivo, F., Maris, J. M., & Wahl, M. L. (2006). The H+-linked monocarboxylate transporter (MCT1/SLC16A1): A potential therapeutic target for high-risk neuroblastoma. Mol. Pharmacol., 70, 2108–2115.
Froberg, M. K., Gerhart, D. Z., Enerson, B. E., Manivel, C., Guzman-Paz, M., Seacotte, N., & Drewes, L. R. (2001). Expression of monocarboxylate transporter MCT1 in normal and neoplastic human CNS tissues. Membr. Cell. Biophys. Bioch., 12, 0959.
Funes, J. M., Quintero, M., Henderson, S., Martinez, D., Qureshi, U., Westwood, C., Clements, M. O., Bourboulia, D., Pedley, R. B., Moncada, S., & Boshoff, C. (2007). Transformation of human mesenchymal stem cells increases their dependency on oxidative phosphorylation for energy production. Proc. Natl. Acad. Sci. USA, 104, 6223–6228.
Gatenby, R. A., & Gillies, R. J. (2004). Why do cancers have high aerobic glycolysis? Nat. Rev., Cancer, 4, 891–899.
Grillon, E., Farion, R., Fablet, K., De Waard, M., Tse, C. M., Donowitz, M., Remy, C., & Coles, J. A. (2011). The spatial organization of proton and lactate transport in a rat brain tumor. PLOS One, 6, e17316.
Halestrap, A. P., & Price, N. T. (1999). The proton-linked monocarboxylate transporter (MCT) family: structure, function and regulation. Biochem. J., 343, 281–299.
Hanahan, D., & Weinberg, R. A. (2011). Hallmarks of cancer: the next generation. Cell, 144, 646–674.
Izumi, H., Takahashi, M., Uramoto, H., Nakayama, Y., Oyama, T., Wang, K.-Y., Sasaguri, Y., Nishizawa, S., & Kohno, K. (2010). Monocarboxylate transporters 1 and 4 are involved in the invasion activity of human lung cancer cells. Cancer Sci., 105, 1007–1013.
Kennedy, K. M., & Dewhrist, M. W. (2010). Tumor metabolism of lactate: the influence and therapeutic potential for MCT and CD147 regulation. Future Oncol., 6, 127–148.
Koppenol, W. H., Bounds, P. L., & Dang, C. V. (2011). Otto Warburg’s contributions to current concepts of cancer metabolism. Nat. Rev., Cancer, 11, 325–337.
Kroemer, G., & Pouyssegur, J. (2008). Tumor cell metabolism: cancer’s achilles’ heel. Cancer Cell, 13, 472–482.
Mankoff, D. A., Eary, J. F., Link, J. M., Muzi, M., Rajendran, J. G., Spence, A. M., & Krohn, K. A. (2007). Tumor-specific positron emission tomography imaging in patients: [18F] fluorodeoxyglucose and beyond. Clin. Cancer Res., 13, 3460–3469.
Mathupala, S. P., Parajuli, P., & Sloan, A. E. (2004). Silencing of monocarboxylate transporters via siRNA inhibits glycolysis and induces cell death in malignant glioma: An in vitro study. Neurosurgery, 55, 1410–1419.
Mathupala, S. P., Colen, C. B., Parajuli, P., & Sloan, A. E. (2007). Lactate and malignant tumors: A therapeutic target at the end stage of glycolysis. J. Bioenerg. Biomembr., 39, 73–77.
Mathupala, S., Ko, Y. H., & Pedersen, P. L. (2010). The pivotal roles of mitochondria in cancer: Warburg and beyond and encouraging prospects for effective therapies. Biochim. Biophys. Acta, 1797, 1225–1230.
McCarthy, N. (2009). Metabolism: room to breathe. Nat. Rev., Cancer, 9, 13.
Michelakis, E. D., Sutendra, G., Dromparis, P., Webster, L., Haromy, A., Niven, E., Maguire, C., Gammer, T.-L., Mackey, J. R., Fulton, D., Abdulkarim, B., McMurtry, M. S., & Petruk, K. C. (2010). Metabolic modulation of glioblastoma with dichloroacetate. Sci. Transl. Med., 2(31), 31ra34.
Moreno-Sánchez, R., Rodríguez-Enríquez, S., Marín-Hernández, A., & Saavedra, E. (2007). Energy metabolism in tumor cells. FEBS J., 274, 1393–1418.
Morris, M. E., & Felmlee, M. A. (2008). Overview of the proton-coupled MCT family of transporters. AAPS J., 10, 311–321.
Pavlides, S., Whitaker-Menezes, D., Castello-Cros, R., Flomenberg, N., Witkiewicz, A. K., Frank, P. G., Casimiro, M. C., Wang, C., Fortina, P., Addya, S., Pestell, R. G., Martinez-Outschoorn, U. E., Sotgia, F., & Lisanti, M. P. (2009). The reverse Warburg effect: Aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle, 8, 3984–4001.
Pavlides, S., Tsirigos, A., Vera, I., Flomenberg, N., Frank, P. G., Casimiro, M. C., Wang, C., Pestell, R. G., Martinez-Outschoorn, U. E., Howell, A., Sotgia, F., & Lisanti, M. P. (2010). Transcriptional evidence for the “Reverse Warburg Effect” in human breast cancer tumor stroma and metastasis: similarities with oxidative stress, inflammation, Alzheimer’s disease, and “Neuron-Glia Metabolic Coupling”. Aging, 2, 185–199.
Pérez-García, V. M., Calvo, G. F., Belmonte-Beitia, J., Diego, D., & Pérez-Romasanta, L. (2011). Bright solitary waves in malignant gliomas. Phys. Rev. E, 84, 021921.
Pinheiro, C., Longatto-Filho, A., Scapulatempo, C., Ferreira, L., Martins, S., Pellerin, L., Rodrigues, M., Alves, V. A. F., Schmitt, F., & Baltazar, F. (2008). Increased expression of monocarboxylate transporters 1, 2, and 4 in colorectal carcinomas. Virchows Arch., 452, 139–146.
Pinheiro, C., Albergaria, A., Paredes, J., Sousa, B., Dufloth, R., Vieira, D., Schmitt, F., & Baltazar, F. (2010a). Monocarboxylate transporter 1 is up-regulated in basal-like breast carcinoma. Histopathology, 56, 860–867.
Pinheiro, C., Reis, R. M., Ricardo, S., Longatto-Filho, A., Schmitt, F., & Baltazar, F. (2010b). Expression of monocarboxylate transporters 1, 2, and 4 in human tumours and their association with CD147 and CD44. J. Biomed. Biotechnol., 2010, 427694.
Rossignol, R., Gilkerson, R., Aggeler, R., Yamagata, K., Remington, S. J., & Capaldi, R. A. (2004). Energy substrate modulates mitochondrial structure and oxidative capacity in cancer cells. Cancer Res., 64, 985–993.
Sauer, L. A., Stayman, J. W., & Dauchy, R. T. (1987). Amino acid, glucose, and lactic acid utilization in vivo by rat tumors. Cancer Res., 42, 4090–4097.
Simpson, I. A., Carruthers, A., & Vannucci, S. J. (2007). Supply and demand in cerebral energy metabolism: The role of nutrient transporters. J. Cereb. Blood Flow Metab., 27, 1766–1791.
Smallbone, K., Gatenby, R. A., & Maini, P. K. (2008). Mathematical modelling of tumour acidity. J. Theor. Biol., 255, 106–112.
Sonveaux, P., Végran, F., Schroeder, T., Wergin, M. C., Verrax, J., Rabbani, Z. N., De Saedeleer, C. J., Kennedy, K. M., Diepart, C., Jordan, B. F., Kelley, M. J., Gallez, B., Wahl, M. L., Feron, O., & Dewhirst, M. W. (2008). Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J. Clin. Invest., 118, 3930–3942.
Sun, R. C., Fadia, M., Dahlstrom, J. E., Parish, C.R., Board, P.G., & Blackburn, A.C. (2009). Reversal of the glycolytic phenotype by dichloroacetate inhibits metastatic breast cancer cell growth in vitro and in vivo. Breast Cancer Res. Treat., 120, 253–260.
Tennant, D. A., Durán, R. V., & Gottlieb, E. (2010). Targeting metabolic transformation for cancer therapy. Nat. Rev., Cancer, 10, 267–277.
Terpstra, M., Gruetter, R., High, W. B., Mescher, M., DelaBarre, L., Merkle, M., & Garwood, M. (1998). Magnetic resonance spectroscopy lactate turnover in rat glioma measured by in vivo nuclear magnetic resonance spectroscopy. Cancer Res., 58, 5083–5088.
Vander Heiden, M. G., Cantley, L. C., & Thompson, C. B. (2009). Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science, 324, 1029–1033.
Vaupel, P., Thews, O., & Hoeckel, M. (2001). Treatment resistance of solid tumors role of hypoxia and anemia. Rev. Med. Oncol., 18(4), 243–259.
Végran, F., Boidot, R., Michiels, C., Sonveaux, P., & Feron, O. (2011). Lactate influx through the endothelial cell monocarboxylate transporter MCT1 supports an NF-kappaB/IL-8 pathway that drives tumor angiogenesis. Cancer Res., 71, 2550–2560.
Venkatasubramanian, R., Henson, M. A., & Forbes, N. S. (2006). Incorporating energy metabolism into a growth model of multicellular tumor spheroids. J. Theor. Biol., 242, 440–453.
Voisin, P., Bouchaud, V., Merle, M., Diolez, P., Duffy, L., Flint, K., Franconi, J.-M., & Bouzier-Sore, A.-K. (2010). Microglia in close vicinity of glioma cells: correlation between phenotype and metabolic alterations. Front. Neuroenerg., 2, 131.
Walenta, S., & Mueller-Klieser, W. F. (2004). Lactate: mirror and motor of tumor malignancy. Semin. Radiat. Oncol., 14, 267–274.
Warburg, O., Posener, K., & Negelein, E. (1924). Über den Stoffwechsel der Tumoren. Biochem. Z., 152, 319–344.
Weissleder, R., & Pittet, M. J. (2008). Imaging in the era of molecular oncology. Nature, 452, 580–589.
Wolf, A., Agnihotri, S., Micallef, J., Mukherjee, J., Sabha, N., Cairns, R., Hawkins, C., & Guha, A. (2011). Hexokinase 2 is a key mediator of aerobic glycolysis and promotes tumor growth in human glioblastoma multiforme. J. Exp. Med., 208, 313–326.
Acknowledgements
We wish to thank P. Melgar and R. Sánchez-Prieto (CRIB, UCLM), and L. Pérez-Romasanta (HGCR) for fruitful discussions. This work has been supported by grants MTM2009-13832 (Ministerio de Ciencia e Innovación, Spain), PEII11-0178-4092 (Junta de Comunidades de Castilla-La Mancha, Spain) and the James S. McDonnell Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mendoza-Juez, B., Martínez-González, A., Calvo, G.F. et al. A Mathematical Model for the Glucose-Lactate Metabolism of in Vitro Cancer Cells. Bull Math Biol 74, 1125–1142 (2012). https://doi.org/10.1007/s11538-011-9711-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-011-9711-z