Abstract
Background
There is limited evidence regarding immune-related adverse events (irAEs) in Asian cancer patients treated with antibodies directed against programmed cell death-1 (PD-1) or programmed cell death-ligand 1 (PD-L1).
Objective
This study aimed to investigate the clinical patterns and prognostic significance of grade 1–2 and grade ≥ 3 irAEs by PD-1/PD-L1 inhibitors in cancer patients using real-world clinical data.
Patients and Methods
We conducted a retrospective study of cancer patients who received pembrolizumab, nivolumab, or atezolizumab at a tertiary hospital in South Korea. Incidence, time to onset, and grade 1–2 and grade ≥ 3 irAE risk factors were analyzed from medical records. The association of irAE severity with progression-free survival (PFS) and prognostic factors for PFS were evaluated.
Results
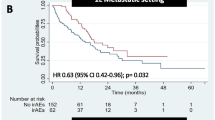
Among a total of 431 patients, irAEs occurred in 45.2%, and 9.5% were grade ≥ 3 irAEs. There were no significant differences in the median time to onset based on severity. Risk factors for the development of grade ≥ 3 irAEs were the presence of autoimmune disorders or diabetes mellitus. The median PFS was significantly different at 13.20, 9.00 and 4.17 months for the grade 1–2, grade ≥ 3, and no irAE groups, respectively. An increase in administration cycles was associated with a reduced risk of progression in patients with grade 1–2 and grade ≥ 3 irAEs.
Conclusions
The development of grade ≥ 3 irAEs was affected by comorbidities and associated with improved PFS compared with those without irAEs. Our findings identified the real-world epidemiology, risk factors, and prognostic significance of irAEs, which may guide treatment decisions of PD-1/PD-L1 inhibitors.


Similar content being viewed by others
References
Ramos-Casals M, Brahmer JR, Callahan MK, Flores-Chávez A, Keegan N, Khamashta MA, et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers. 2020;6(1):38. https://doi.org/10.1093/annonc/mdx286.
U.S. Food and Drug Administration. Drugs@FDA: FDA-Approved Drugs. https://www.accessdata.fda.gov/scripts/cder/daf/. Accessed 3 May 2022.
European Medicines Agency. Medicines. https://www.ema.europa.eu/en/medicines. Accessed 3 May 2022.
Korean Ministry of Food and Drug Safety. Searching for drug information. https://nedrug.mfds.go.kr/searchDrug. Accessed 3 May 2022.
Baxi S, Yang A, Gennarelli RL, Khan N, Wang Z, Boyce L, Korenstein D. Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: systematic review and meta-analysis. BMJ. 2018;360: k793. https://doi.org/10.1136/bmj.k793.
Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378(2):158–68. https://doi.org/10.1056/NEJMra1703481.
Khoja L, Day D, Wei-Wu Chen T, Siu LL, Hansen AR. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Ann Oncol. 2017;28(10):2377–85. https://doi.org/10.1093/annonc/mdx286.
Conroy M, Naidoo J. Immune-related adverse events and the balancing act of immunotherapy. Nat Commun. 2022;13(1):392. https://doi.org/10.1038/s41467-022-27960-2.
Petrelli F, Grizzi G, Ghidini M, Ghidini A, Ratti M, Panni S, et al. Immune-related adverse events and survival in solid tumors treated with immune checkpoint inhibitors: a systematic review and meta-analysis. J Immunother. 2020;43(1):1–7. https://doi.org/10.1097/cji.0000000000000300.
Park R, Lopes L, Saeed A. Anti-PD-1/L1-associated immune-related adverse events as harbinger of favorable clinical outcome: systematic review and meta-analysis. Clin Transl Oncol. 2021;23(1):100–9. https://doi.org/10.1007/s12094-020-02397-5.
Zhao JJ, Wen XZ, Ding Y, Li DD, Zhu BY, Li JJ, Weng DS, Zhang X, Zhang XS. Association between immune-related adverse events and efficacy of PD-1 inhibitors in Chinese patients with advanced melanoma. Aging. 2020;12(11):10663–75. https://doi.org/10.18632/aging.103285.
Wang W, Gu X, Wang L, Pu X, Feng H, Xu C, et al. The prognostic impact of mild and severe immune-related adverse events in non-small cell lung cancer treated with immune checkpoint inhibitors: a multicenter retrospective study. Cancer Immunol Immunother. 2022;71(7):1693–703.
Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2018;36(17):1714–68. https://doi.org/10.1200/jco.2017.77.6385.
Kalinich M, Murphy W, Wongvibulsin S, Pahalyants V, Yu KH, Lu C, et al. Prediction of severe immune-related adverse events requiring hospital admission in patients on immune checkpoint inhibitors: study of a population level insurance claims database from the USA. J Immunother Cancer. 2021;9(3):e001935. https://doi.org/10.1136/jitc-2020-001935.
Ruste V, Goldschmidt V, Laparra A, Messayke S, Danlos FX, Romano-Martin P, et al. The determinants of very severe immune-related adverse events associated with immune checkpoint inhibitors: a prospective study of the French REISAMIC registry. Eur J Cancer. 2021;158:217–24. https://doi.org/10.1016/j.ejca.2021.08.048.
Cortellini A, Friedlaender A, Banna GL, Porzio G, Bersanelli M, Cappuzzo F, et al. Immune-related adverse events of pembrolizumab in a large real-world cohort of patients with NSCLC with a PD-L1 expression ≥ 50% and their relationship with clinical outcomes. Clin Lung Cancer. 2020;21(6):498–508. https://doi.org/10.1016/j.cllc.2020.06.010.
Huang Y, Soon YY, Aminkeng F, Tay SH, Ang Y, Kee ACL, Goh BC, Wong ASC, Soo RA. Risk factors for immune-related adverse events from anti-PD-1 or anti-PD-L1 treatment in an Asian cohort of nonsmall cell lung cancer patients. Int J Cancer. 2022;150(4):636–44. https://doi.org/10.1002/ijc.33822.
Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018;4(12):1721–8. https://doi.org/10.1001/jamaoncol.2018.3923.
Shankar B, Zhang J, Naqash AR, Forde PM, Feliciano JL, Marrone KA, et al. Multisystem immune-related adverse events associated with immune checkpoint inhibitors for treatment of non-small cell lung cancer. JAMA Oncol. 2020;6(12):1952–6. https://doi.org/10.1001/jamaoncol.2020.5012.
U.S. Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. 2017. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_60. Accessed 21 Mar 2018.
Puzanov I, Diab A, Abdallah K, Bingham CO, Brogdon C, Dadu R, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 2017;5(1):95. https://doi.org/10.1186/s40425-017-0300-z.
Thompson JA, Schneider BJ, Brahmer J, Andrews S, Armand P, Bhatia S, et al. Management of immunotherapy-related toxicities, version 1.2019. J Natl Compr Canc Netw. 2019;17(3):255–89. https://doi.org/10.6004/jnccn.2019.0013.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47. https://doi.org/10.1016/j.ejca.2008.10.026.
Zhang Q, Wang W, Yuan Q, Li L, Wang YC, Chi CZ, Xu CH. Correlation between immune-related adverse events and the efficacy of PD-1/PD-L1 inhibitors in the treatment of non-small cell lung cancer: systematic review and meta-analysis. Cancer Chemother Pharmacol. 2022;89(1):1–9. https://doi.org/10.1007/s00280-021-04375-2.
Suo A, Chan Y, Beaulieu C, Kong S, Cheung WY, Monzon JG, Smylie M, Walker J, Morris D, Cheng T. Anti-PD1-induced immune-related adverse events and survival outcomes in advanced melanoma. Oncologist. 2020;25(5):438–46. https://doi.org/10.1634/theoncologist.2019-0674.
Gülave B, Hew MN, de Groot JS, Rodwell L, Teerenstra S, Fabriek BO. High body mass index and pre-existing autoimmune disease are associated with an increased risk of immune-related adverse events in cancer patients treated with PD-(L)1 inhibitors across different solid tumors. ESMO Open. 2021;6(3):100107. https://doi.org/10.1016/j.esmoop.2021.100107.
Kehl KL, Yang S, Awad MM, Palmer N, Kohane IS, Schrag D. Pre-existing autoimmune disease and the risk of immune-related adverse events among patients receiving checkpoint inhibitors for cancer. Cancer Immunol Immunother. 2019;68(6):917–26. https://doi.org/10.1007/s00262-019-02321-z.
Shi B, Du X, Wang Q, Chen Y, Zhang X. Increased PD-1 on CD4(+)CD28(-) T cell and soluble PD-1 ligand-1 in patients with T2DM: association with atherosclerotic macrovascular diseases. Metabolism. 2013;62(6):778–85. https://doi.org/10.1016/j.metabol.2012.12.005.
Eun Y, Kim IY, Sun JM, Lee J, Cha HS, Koh EM, Kim H, Lee J. Risk factors for immune-related adverse events associated with anti-PD-1 pembrolizumab. Sci Rep. 2019;9(1):14039. https://doi.org/10.1038/s41598-019-50574-6.
Del Rivero J, Cordes LM, Klubo-Gwiezdzinska J, Madan RA, Nieman LK, Gulley JL. Endocrine-related adverse events related to immune checkpoint inhibitors: proposed algorithms for management. Oncologist. 2020;25(4):290–300. https://doi.org/10.1634/theoncologist.2018-0470.
Tang SQ, Tang LL, Mao YP, Li WF, Chen L, Zhang Y, Guo Y, Liu Q, Sun Y, Xu C, Ma J. The pattern of time to onset and resolution of immune-related adverse events caused by immune checkpoint inhibitors in cancer: a pooled analysis of 23 clinical trials and 8,436 patients. Cancer Res Treat. 2021;53(2):339–54. https://doi.org/10.4143/crt.2020.790.
Suzman DL, Pelosof L, Rosenberg A, Avigan MI. Hepatotoxicity of immune checkpoint inhibitors: an evolving picture of risk associated with a vital class of immunotherapy agents. Liver Int. 2018;38(6):976–87. https://doi.org/10.1111/liv.13746.
González-Rodríguez E, Rodríguez-Abreu D. Immune checkpoint inhibitors: review and management of endocrine adverse events. Oncologist. 2016;21(7):804–16. https://doi.org/10.1634/theoncologist.2015-0509.
Hasegawa S, Ikesue H, Nakao S, Shimada K, Mukai R, Tanaka M, et al. Analysis of immune-related adverse events caused by immune checkpoint inhibitors using the Japanese Adverse Drug Event Report database. Pharmacoepidemiol Drug Saf. 2020;29(10):1279–94. https://doi.org/10.1002/pds.5108.
Chen X, Nie J, Dai L, Hu W, Zhang J, Han J, et al. Immune-related adverse events and their association with the effectiveness of PD-1/PD-L1 inhibitors in non-small cell lung cancer: a real-world study from China. Front Oncol. 2021;11:607531. https://doi.org/10.3389/fonc.2021.607531.
Wang PF, Chen Y, Song SY, Wang TJ, Ji WJ, Li SW, Liu N, Yan CX. Immune-related adverse events associated with anti-PD-1/PD-L1 treatment for malignancies: a meta-analysis. Front Pharmacol. 2017;8:730. https://doi.org/10.3389/fphar.2017.00730.
Shimozaki K, Sukawa Y, Sato Y, Horie S, Chida A, Tsugaru K, et al. Analysis of risk factors for immune-related adverse events in various solid tumors using real-world data. Fut Oncol. 2021;17(20):2593–603. https://doi.org/10.2217/fon-2020-0861.
Haratani K, Hayashi H, Chiba Y, Kudo K, Yonesaka K, Kato R, et al. Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol. 2018;4(3):374–8. https://doi.org/10.1001/jamaoncol.2017.2925.
Di Maio M, Perrone F, Conte P. Real-world evidence in oncology: opportunities and limitations. Oncologist. 2020;25(5):e746–52. https://doi.org/10.1634/theoncologist.2019-0647.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This research was supported by a Grant (22183MFDS499) from the Ministry of Food and Drug Safety in 2022.
Conflicts of interest
Su Jeong Song, Yun-Kyoung Song, Mihwa Jang, Eunjeong Shin, Sung Yun Suh, Yoon Sook Cho, Ju-Yeun Lee, and Jung Mi Oh declare that they have no conflicts of interest that might be relevant to the contents of this manuscript.
Availability of data and material
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. The data are not publicly available due to privacy or ethical restrictions.
Ethics approval
This retrospective chart review study involving human participants was in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study was approved by the Institutional Review Board of the SNUH (IRB No. 1903-135-1020, April 1, 2019).
Consent to participate
Informed consent was waived due to the retrospective design of the study.
Consent for publication
Not applicable.
Code availability
Not applicable.
Author contributions
SJS: conceptualization, data curation, formal analysis, methodology, project administration, writing—original draft; Y-KS: conceptualization, formal analysis, software; validation; visualization; writing—original draft; MJ: investigation, formal analysis; EJS: resources, writing—review & editing; SYS: resources, writing—review & editing; YSC: resources, writing—review & editing; J-YL: conceptualization, methodology, writing—review & editing; JMO: conceptualization; funding acquisition; methodology; supervision; writing—review & editing. All authors read and approved the final manuscript.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Song, S.J., Song, YK., Jang, M. et al. Prognostic Significance of the Severity of Immune-Related Adverse Events in Advanced Cancer Patients Treated with PD-1/PD-L1 Inhibitors: A Real-World Data Analysis. Targ Oncol 18, 147–158 (2023). https://doi.org/10.1007/s11523-022-00936-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-022-00936-4