Abstract
Background
KRAS is the most frequently mutated gene in non-small cell lung cancer (NSCLC), however conflicting data are available on its role as a biomarker.
Objective
The aim of our work was to investigate the impact of KRAS mutations on response and survival outcomes in advanced non-squamous NSCLC patients treated with immune checkpoint inhibitors alone or in combination with chemotherapy.
Patients and Methods
We retrospectively identified 119 patients, most of whom (58%) were KRAS wild type. For each patient we evaluated overall survival (OS), progression-free survival (PFS), and disease control rate (DCR). An exploratory analysis was performed among KRAS mutated patients to investigate the impact of specific KRAS mutations on response and survival outcomes.
Results
After a median follow-up of 10.3 months, the median OS was 14.9 months (95% confidence interval [CI] 7.6–22.7) in wild-type KRAS patients versus 14.7 months (95% CI 8.0–19.5) in mutated KRAS patients (p = 0.529). No differences were detected between the two groups in terms of PFS and DCR. Patients with a KRAS G12C mutation reported survival and response outcomes that were not statistically different from those of patients with other KRAS mutations.
Conclusion
Our data confirmed that KRAS mutational status is not associated with survival and response outcomes in advanced non-squamous NSCLC patients treated with immunotherapy alone or combined with chemotherapy.
Similar content being viewed by others
In this retrospective analysis, survival and response outcomes of patients with KRAS-mutant non-small cell lung cancer (NSCLC) were not statistically different from those of patients with wild-type KRAS NSCLC receiving immune checkpoint inhibitors alone or combined with chemotherapy for advanced disease. |
Among mutated KRAS patients, no differences were found according to the subtype of mutation (G12C vs. others). |
KRAS mutational status was shown to be neither a prognostic nor predictive biomarker in patients with advanced non-squamous NSCLC treated with immunotherapy or chemoimmunotherapy. |
1 Introduction
The prognosis of patients with non-small cell lung cancer (NSCLC) has significantly improved in recent years thanks to advances in molecular diagnostics and targeted treatments. A comprehensive genomic screening may identify aberrations in oncogenes, including EGFR, ALK, ROS1, BRAF, and MET, and specific inhibitors for all these targets are now available [1]. Mutations of Kirsten rat sarcoma viral oncogene homolog (KRAS) are the most frequent genomic alteration in NSCLC, accounting for approximately 30% of non-squamous NSCLCs [1]. KRAS encodes an intracellalur protein belonging to the family of guanosine triphosphate (GTP)-binding proteins, and it is responsible for the control of cellular signaling transduction and the regulation of cell proliferation. After GTP binds to mutated KRAS protein, its constitutive activation triggers downstream effectors, including EGFR, RAF, MEK, PI3K and AKT, leading to uncontrolled tumour cell proliferation and survival [1,2,3]. KRAS mutations are missense and result in amino acid changes in codons 12, 13 or 61. The most common amino acid change is from a glycine to a cysteine in codon 12 (G12C) that is detected in 13% of lung cancers; others are G12A, G12D, G12R, G12V, G13D, Q61L, and Q61H [4, 5]. KRAS G12C and G12V are more common in smokers, while G12D is the most prevalent KRAS codon alteration in former or non-smokers [6].
While several agents were developed to target most of the gene mutations in NSCLC patients, until recently, no targeted therapy was available for mutated KRAS patients. Chemotherapy and immunotherapy represent two options for this subgroup of patients, who are often smokers and with higher PD-L1 expression levels [7, 8]. Moreover, genomic analyses showed that KRAS-mutant tumours are heterogeneous because of concomitant mutations such as TP53, CDKN2A/2B, STK11, and KEAP1, which give the tumour different biological properties and therapeutic vulnerability [1, 6, 9, 10]. For example, concomitant KRAS and TP53 mutations, found in about 40% of KRAS-mutant patients, are associated with increased tumour cell proliferation and inflammation, and higher expression levels of PD-L1, resulting in higher response rates to immunotherapy [6, 11]. These factors may contribute to the sensitivity of mutated KRAS tumours to immune checkpoint inhibitors (ICIs). In the multicentric retrospective IMMUNOTARGET study, ICIs were more effective in mutated KRAS patients than in other subgroups of oncogene-addicted tumours. In 271 KRAS-mutant patients, the response rate was 26%, PFS was 3.2 months (95% confidence interval [CI] 2.7–4.5), and OS was 13.5 months (95% CI 9.4–15.6); the rate of rapid progression (within 2 months) was lower (36%) than that reported in the EGFR (44.8%), ALK (45.5%), or ROS1 (42.9%) populations [12].
The aim of our study was to retrospectively evaluate the impact of KRAS mutations on response and survival outcomes in advanced nonsquamous NSCLC patients treated with ICIs alone or in combination with chemotherapy.
2 Methods
2.1 Patients and Methods
We conducted a retrospective cohort study, analyzing a consecutive series of patients with a histological diagnosis of advanced non-squamous NSCLC and known KRAS mutational status who had received at least one cycle of ICI therapy (atezolizumab, pembrolizumab or nivolumab) at Santa Chiara Hospital, Trento, from March 2017 to August 2021. Patients could receive immunotherapy alone or in combination with chemotherapy in any line of treatment, according to daily clinical practice, and had to have a minimum follow-up of 6 months. KRAS mutations were tested by diagnostic methods available at our Institute (sequenom, real time PCR, next-generation sequencing), while PD-L1 expression levels were analyzed on tumour cells by immunohistochemistry, according to the currently used assay.
We collected the following baseline patient characteristics from the clinical records: sex, date of metastatic disease diagnosis, age at diagnosis, smoking status, number of comorbidities, Eastern Cooperative Oncology Group (ECOG) performance status, histologic subtype, stage, number and type of metastatic sites, and biomolecular phenotype, including PD-L1 expression levels and mutational status of EGFR/ALK/ROS1/KRAS/BRAF/other genes.
The following data on ICI-based treatment were collected: type of ICI (atezolizumab, pembrolizumab or nivolumab), possible concomitant administration of chemotherapy, date of first administration, best response to treatment, date and reason of progression/discontinuation, number of cycles received, palliative radiotherapy treatments, and subsequent lines of treatment. Patients were radiologically monitored according to local clinical practice. The response was evaluated according to Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1. Lastly, vital status (alive or dead) and date of death/last follow-up were collected.
This study was approved by the APSS Ethical Committee, Trento.
2.2 Statistical Analysis
Descriptive statistics were used to report patient characteristics: median with interquartile range was used to report continuous variables, and frequency (percentage) was used for categorical variables.
OS was calculated from the date of metastatic disease diagnosis until death due to any cause or the date of the last follow-up for censored patients. In patients receiving an ICI as first line, PFS was calculated from the date of metastatic disease diagnosis until disease progression or death due to any cause or the date of the last follow-up for censored patients; in patients receiving an ICI as a subsequent line of treatment, PFS was calculated from the date of disease progression to previous treatment until disease progression or death due to any cause or the date of the last follow-up for censored patients.
Disease control rate (DCR) was defined as the sum of complete response rate, partial response rate, and stable disease rate.
Kaplan–Meier survival curves were used to estimate median OS and PFS, including 95% CI, and stratified by KRAS mutational status (mutated vs. wild type). Differences were tested via the log-rank test. A Cox proportional hazards model was used to develop multivariable prediction models for OS and PFS. A backward variable selection method with a type I error criterion of 0.05 was used to select factors significantly affecting PFS and OS. An exploratory analysis was performed among mutated patients to investigate the impact of specific KRAS mutations on response and survival outcomes. Statistical analyses were performed using R software version 4.1.2 (R Foundation for Statistical Computing, Vienna, Austria) [13].
3 Results
We identified a consecutive series of 119 patients treated with an ICI alone or in combination with chemotherapy from March 2017 to August 2021 at Santa Chiara Hospital, Trento.
Baseline patient characteristics are shown in Table 1. Most patients were male (65.5%) and a current or former smoker (84.1%) with adenocarcinoma (89.9%) in stage IV (100%). All were tested for KRAS mutations, with a result of wild type or mutated in 69 and 50 patients, respectively. In the overall population, the median follow-up was 10.3 months (range 0.6–57.3). The median duration of the immunotherapy treatment was 6.2 months (range 0.3–57.3).
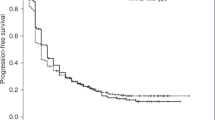
No statistically significant difference in OS was found between wild type and mutated KRAS patients: median OS was 14.9 months (95% CI 7.6–22.7) in wild-type KRAS patients versus 14.7 months (95% CI 8.0–19.5) in mutated KRAS patients; p = 0.529 (Fig. 1). Similarly, no statistically significant differences in terms of PFS were reported: median PFS was 7.2 months (95% CI 3.5–14.5) in wild-type KRAS patients versus 8.8 months (95% CI 4.4–14.7) in mutated KRAS patients; p = 0.768 (Fig. 2). The DCR was 55% and 64% in the wild type and mutated KRAS groups, respectively. This confirmed that the control rate was not significantly associated with KRAS status (p = 0.642) (Table 2).
At multivariable analysis, brain metastases (HR 2.11, 95% CI 1.29–3.45; p = 0.002) and nivolumab treatment (0.36, 95% CI 0.16–0.79; p = 0.011) were independently associated with OS, while brain metastases (2.14, 95% CI 1.31–3.51; p = 0.002) and immune-chemotherapy treatment (0.40, 95% CI 0.19–0.85; p = 0.017) were independently associated with PFS (Table 3). Patients with brain involvement reported a significantly shorter OS and PFS, while those treated with immunotherapy plus chemotherapy reported a significantly longer PFS.
No statistically significant difference was found in terms of PFS and OS between patients with PS 0 or PS 2, which was likely due to the small number of patients with PS 2 (only 4) versus PS 0 (92).
We collected the KRAS mutation subtypes and evaluated their impact on survival. Among mutated KRAS patients, 26 (52%) had p.G12C, while 24 (48%) patients had other mutations (p.G12A, p.G12D, p.G12S, p.G12V, p.G13D, pQ61H, pQ61L). Median OS was 11 months (95% CI 5.6–19.5) in KRAS G12C patients versus 17 months (95% CI 6.3–31.1) in patients with other KRAS mutations; p = 0.448 (Fig. 3). Median PFS was 6 months (95% CI 3.7–14.6) in KRAS G12C patients versus 11 months (95% CI 3.9–17) in patients with other KRAS mutations; p = 0.609 (Fig. 4). DCR was similar between the two groups: 61.5% in the KRAS G12C group versus 66.7% in the other mutation groups (p = 0.912).
Data on subsequent treatments were not reported, however no patient had ever received a specific KRAS inhibitor because this class of drugs was not available at our institute during the time the patients were treated.
4 Discussion
This study investigated the prognostic role of KRAS mutational status in advanced NSCLC patients treated with an ICI alone or combined with chemotherapy. After a median follow-up of 10.3 months, we did not find any differences between wild type and mutated KRAS patients in terms of survival or response outcomes; OS, PFS and DCR were similar between the two groups.
Several meta-analyses have been performed on this topic, leading to conflicting results; two of these meta-analyses failed to demonstrate an impact of KRAS mutational status on survival of NSCLC patients treated with ICIs [14, 15]. More recently, another meta-analysis was performed on six studies, which compared an anti-PD-(L)1 with or without chemotherapy and chemotherapy alone. The authors found that in 386 KRAS-mutant patients, anti-PD-(L)1 plus chemotherapy prolonged OS (HR 0.59, 95% CI 0.49–0.72; p < 0.00001) compared with chemotherapy alone, regardless of the treatment line. Moreover, OS was significantly longer in mutated KRAS patients than wild-type KRAS patients (p = 0.001) [16]. Finally, a meta-analysis regarding the activity of ICIs in oncogene-addicted NSCLC patients did not demonstrate significant differences in terms of the response rate between mutated and wild-type KRAS patients (odds ratio 1.54, 95% CI 0.81–2.92; p = 0.19) [17].
Some real-world retrospective studies tried to clarify the role of KRAS status in NSCLC patients treated with ICIs, again with conflicting results. A Swiss study including 38 patients treated with nivolumab, pembrolizumab or atezolizumab retrospectively reported the efficacy of immunotherapy in mutant KRAS NSCLC patients. DCR, PFS and OS were higher in mutant patients than in wild-type patients: 81% vs. 71%, 13.6 vs. 11.3 months, and 18.5 vs. 17.7 months, respectively [18]. Conversely, another retrospective study did not detect differences in terms of PFS (4.6 vs. 3.3 months; p = 0.58) and OS (8.1 vs. 13 months; p = 0.38) between 43 mutant KRAS and 117 non-matched wild-type KRAS NSCLC patients treated with ICIs. At multivariate analysis, only ECOG PS 2 was associated with a higher risk of death (HR 3.14, 95% CI 1.42–6.92; p = 0.005) [19]. Similarly, also the largest real-world retrospective study on advanced lung adenocarcinoma patients receiving first-line pembrolizumab failed to confirm an impact of KRAS status on OS (HR 1.03, 95% CI 0.83–1.29), reporting similar survival outcomes between wild-type and mutant KRAS patients (the latter representing 57% of 595 patients) [20].
Our results are in line with those discussed shown: wild-type and mutated KRAS patients demonstrated similar results in terms of OS (14.9 months vs. 14.7 months; p = 0.529), PFS (7.2 months vs. 8.8 months; p = 0.768) and DCR (55% and 64%; p = 0.642).
In our study, at multivariable analysis, brain metastases were independently associated with survival, showing a significantly shorter OS and PFS, likely due to unfavourable prognosis of this subgroup of patients. Instead, patients treated with immunotherapy plus chemotherapy reported a significantly longer PFS, likely related to higher efficacy of combination treatment in mutated KRAS patients.
We also performed a subgroup analysis to explore the impact of KRAS mutation subtype on response and survival outcomes of patients with advanced NSCLC receiving immunotherapy. We found no statistically significant difference in OS, PFS, or DCR between patients with pG12C mutations and those with other KRAS mutations, confirming previously published data [19]. Results from the literature were in line with our results on the prognostic role of KRAS subtypes. In the IMMUNOTARGET study, PFS was not significantly different between KRAS mutation subtypes: G12C versus other KRAS mutations (p = 0.47); and G12D versus other KRAS mutations (p = 0.40). PFS also was independent of the type of alteration: 2.9 months for transition versus 4.0 months for transversion (p = 0.27). PFS did not show a correlation with smoking or number of previous lines of treatment [12]. On the contrary, the Swiss study found that the PFS in the G12C subgroup was longer (19.1 months) than in other KRAS mutation subtypes (7.8, 9.4, 2.2 and 13.9 months for G13C, G12V, G61H, and other mutations, respectively) [18].
The negative prognostic role of KRAS G12C mutation has also been confirmed in 1014 surgically resected stage I–III lung cancers [21].
The largest retrospective observational study on KRAS mutations identified 743 G12C mutated patients among 7069 patients with advanced NSCLC; survival outcomes were independent of G12C mutations and STK11/KEAP1 co-mutations, which were associated with poorer prognosis [22]. Data in the literature are conflicting in regard to the impact of co-mutations on response to immunotherapy in KRAS patients. Mutated KRAS and TP53 patients were found to better respond to immunotherapy [6, 10, 11], while the co-mutations STK11 and KEAP1, detected in about 7% and 23% of KRAS-mutated patients, respectively, are associated with resistance to immunotherapy [1, 6, 10, 18]. In our study, co-mutations were identified in only 11 patients, which we considered too small a subgroup to perform any analysis of their impact on response and survival outcomes.
The detection of KRAS G12C mutation has become important after the introduction of sotorasib, an irreversible inhibitor of KRAS. Promising activity in heavily pretreated lung cancer patients harboring KRAS G12C was reported in a phase I study published in 2020 [23]. The subsequent phase II trial confirmed its efficacy in patients previously treated with both platinum-based chemotherapy and ICIs: the DCR was 80.6%; the median PFS and OS were 6.8 and 12.5 months, respectively; and G3-4 treatment-related events were 20.6% [24]. The ongoing phase III trial comparing sotorasib with docetaxel will better define the role of sotorasib in the treatment algorithm of KRAS G12C-mutated NSCLC patients (ClinicalTrials.gov identifier: NCT04303780) [25]. Other KRAS inhibitors are being investigated to target KRAS G12C, alone or in combination with chemotherapy or targeted therapies, in order to prevent or delay the development of resistance mechanisms [26].
Our study has some limitations related to the retrospective nature of our research. First, we identified a percentage of KRAS-mutated patients (42%) that is higher than that reported in the literature (about 30%) as well as historically in our institute, where the number of KRAS-mutated patients was generally about 35%. Second, the study population was quite heterogeneous in terms of administered drug and line of treatment; most of the patients (68.9%) received pembrolizumab as first-line treatment, alone or combined with chemotherapy. Third, a longer follow-up and mature OS data are needed to confirm that mutated KRAS patients receiving immunotherapy plus chemotherapy may report a significantly longer survival. Finally, we did not analyse the impact of co-mutations on survival outcomes because they were detected only in 11 patients.
5 Conclusion
Our study confirmed that KRAS mutational status does not negatively impact survival and response outcomes of patients wih advanced non-squamous NSCLC receiving an ICI alone or in combination with chemotherapy. Although previously published data on the prognostic role of KRAS are conflicting, KRAS may not be considered a predictive biomarker of response to immunotherapy.
References
Yang H, Liang SQ, Schmid RA, Peng RW. New horizons in KRAS-mutant lung cancer: dawn after darkness. Front Oncol. 2019;9:953. https://doi.org/10.3389/fonc.2019.00953.
Nagasaka M, Li Y, Sukari A, Ou SI, Al-Hallak MN, Azmi AS. KRAS G12C Game of Thrones, which direct KRAS inhibitor will claim the iron throne? Cancer Treat Rev. 2020;84: 101974. https://doi.org/10.1016/j.ctrv.2020.101974.
Xie M, Xu X, Fan Y. KRAS-mutant non-small cell lung cancer: an emerging promisingly treatable subgroup. Front Oncol. 2021;11: 672612. https://doi.org/10.3389/fonc.2021.672612.
Hunter JC, Manandhar A, Carrosco MA, Gurbani D, Gondi S, Westover KD. Biochemical and structural analysis of common cancer-associated KRAS mutations. Mol Cancer Res. 2015;13(9):1325–35. https://doi.org/10.1158/1541-7786.MCR-15-0203.
Biernacka A, Tsongalis PD, Peterson JD, de Abreu FB, Black CC, Gutmann EJ, et al. The potential utility of re-mining results of somatic mutation testing: KRAS status in lung adenocarcinoma. Cancer Genet. 2016;209:195–8. https://doi.org/10.1016/j.cancergen.2016.03.001.
Davis AP, Cooper WA, Boyer M, Lee JH, Pavlakis N, Kao SC. Efficacy of immunotherapy in KRAS-mutant non-small-cell lung cancer with comutations. Immunotherapy. 2021;13(11):941–52. https://doi.org/10.2217/imt-2021-0090.
Lan B, Ma C, Zhang C, Chai S, Wang P, Ding L, et al. Association between PD-L1 expression and driver gene status in non-small-cell lung cancer: a meta-analysis. Oncotarget. 2018;9(7):7684–99. https://doi.org/10.18632/oncotarget.23969.
Ng TL, Liu Y, Dimou A, Patil T, Aisner DL, Dong Z, et al. Predictive value of oncogenic driver subtype, programmed death-1 ligand (PD-L1) score, and smoking status on the efficacy of PD-1/PD-L1 inhibitors in patients with oncogene-driven non-small cell lung cancer. Cancer. 2019;125(7):1038–49. https://doi.org/10.1002/cncr.31871.
Ghimessy A, Radeczky P, Laszlo V, Hegedus B, Renyi-Vamos F, Fillinger J, et al. Current therapy of KRAS-mutant lung cancer. Cancer Metastasis Rev. 2020;39(4):1159–77. https://doi.org/10.1007/s10555-020-09903-9.
Gu M, Xu T, Chang P. KRAS/LKB1 and KRAS/TP53 co-mutations create divergent immune signatures in lung adenocarcinomas. Ther Adv Med Oncol. 2021;13: 17588359211006950. https://doi.org/10.1177/17588359211006950.
Liu C, Zheng S, Jin R, Wang X, Wang F, Zang R, et al. The superior efficacy of anti-PD-1/PD-L1 immunotherapy in KRAS-mutant non-small cell lung cancer that correlates with an inflammatory phenotype and increased immunogenicity. Cancer Lett. 2020;470:95–105. https://doi.org/10.1016/j.canlet.2019.10.027.
Mazieres J, Drilon A, Lusque A, Mhanna L, Cortot AB, Mezquita L, et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET registry. Ann Oncol. 2019;30(8):1321–8. https://doi.org/10.1093/annonc/mdz167.
R Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2021. https://www.R-project.org/.
Lee CK, Man J, Lord S, Links M, Gebski V, Mok T, et al. Checkpoint inhibitors in metastatic EGFR-mutated non-small cell lung cancer—a meta-analysis. J Thorac Oncol. 2016;12(2):403–7. https://doi.org/10.1016/j.jtho.2016.10.007.
Kim JH, Kim HS, Kim BJ. Prognostic value of KRAS mutation in advanced non-small-cell lung cancer treated with immune checkpoint inhibitors: a meta-analysis and review. Oncotarget. 2017;8(29):48248–52. https://doi.org/10.18632/oncotarget.17594.
Landre T, Justeau G, Assié JB, Chouahnia K, Davoine C, Taleb C, et al. Anti-PD-(L)1 for KRAS-mutant advanced non-small-cell lung cancers: a meta-analysis of randomized-controlled trials. Cancer Immunol Immunother. 2022;71:719–26. https://doi.org/10.1007/s00262-021-03031-1.
Guaitoli G, Tiseo M, Di Maio M, Friboulet L, Facchinetti F. Immune checkpoint inhibitors in oncogene-addicted non-small cell lung cancer: a systematic review and meta-analysis. Transl Lung Cancer Res. 2021;10(6):2890–916. https://doi.org/10.21037/tlcr-20-941.
Torralvo J, Friedlaender A, Achard V, Addeo A. The activity of immune checkpoint inhibition in KRAS mutated non-small cell lung cancer: a single centre experience. Cancer Genom Proteom. 2019;16(6):577–82. https://doi.org/10.21873/cgp.20160.
Gianoncelli L, Spitaleri G, Passaro A, Radice D, Fumagalli C, Del Signore E, et al. Efficacy of anti-PD1/PD-L1 therapy (IO) in KRAS mutant non-small cell lung cancer patients: a retrospective analysis. Anticancer Res. 2020;40(1):427–33. https://doi.org/10.21873/anticanres.13970.
Noordhof AL, DamhuisDamhuis RAM, Hendriks LEL, de Langen AJ, Timens W, Venmans BJW, et al. Prognostic impact of KRAS mutation status for patients with stage IV adenocarcinoma of the lung treated with first-line pembrolizumab monotherapy. Lung Cancer. 2021;155:163–9. https://doi.org/10.1016/j.lungcan.2021.04.001.
Finn SP, Addeo A, Dafni U, Thunnissen E, Bubendorf L, Madsen LB, et al. Prognostic impact of KRAS G12C mutation in patients with NSCLC: results from the European Thoracic Oncology Platform Lungscape Project. J Thorac Oncol. 2021;16(6):990–1002. https://doi.org/10.1016/j.jtho.2021.02.016.
Spira AI, Tu H, Aggarwal S, Hsu H, Carrigan G, Wang X, et al. A retrospective observational study of the natural history of advanced non-small-cell lung cancer in patients with KRAS p.G12C mutated or wild-type disease. Lung Cancer. 2021;159:1–9. https://doi.org/10.1016/j.lungcan.2021.05.026.
Hong DS, Fakih MG, Strickler JH, Desai J, Durm GA, Shapiro GI, et al. KRAS(G12C) inhibition with sotorasib in advanced solid tumors. N Engl J Med. 2020;383(13):1207–17. https://doi.org/10.1056/NEJMoa1917239.
Skoulidis F, Li BT, Dy GK, Price TJ, Falchook GS, Wolf J, et al. Sotorasib for lung cancers with KRAS p.G12C mutation. N Engl J Med. 2021;384(25):2371–81. https://doi.org/10.1056/NEJMoa2103695.
Amgen. Study to compare AMG 510 “Proposed INN Sotorasib” with docetaxel in non small cell lung cancer (NSCLC) (CodeBreak 200). ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04303780.
Reck M, Carbone DP, Garassino M, Barlesi F. Targeting KRAS in non-small-cell lung cancer: recent progress and new approaches. Ann Oncol. 2021;32(9):1101–10. https://doi.org/10.1016/j.annonc.2021.06.001.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No external funding was used in the preparation of this manuscript.
Conflict of Interest
Antonello Veccia, Mariachiara Dipasquale, Stefania Kinspergher, Sara Monteverdi, Salvatore Girlando, Mattia Barbareschi, and Orazio Caffo declare they have no conflicts of interest that might be relevant to the contents of this manuscript.
Ethics Approval
This study was approved by the Ethical Committee of APSS, Trento.
Consent to Participate
Not applicable due to the retrospective nature of the study.
Consent for Publication
All authors gave their consent for publication.
Availability of Data and Material
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
Author Contributions
AV and OC contributed to the study conception and design, and material preparation, data collection and analysis. The first draft of the manuscript was written by AV and OC, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Veccia, A., Dipasquale, M., Kinspergher, S. et al. Impact of KRAS Mutations on Clinical Outcomes of Patients with Advanced Non-Squamous Non-Small Cell Lung Cancer Receiving Anti-PD-1/PD-L1 Therapy. Targ Oncol 18, 129–138 (2023). https://doi.org/10.1007/s11523-022-00934-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-022-00934-6