Abstract
Background
Data regarding the efficacy and safety profiles of immune checkpoint inhibitors (ICIs) for metastatic renal cell carcinoma (mRCC) trial-ineligible patients in the real world remain unclear.
Objectives
The aim of this study was to clarify the impact of trial eligibility on ICI-based combination therapy for mRCC.
Patients and Methods
We collected clinical data of mRCC patients receiving ICIs since 2016, and 222 patients were registered. Among these patients, we evaluated 93 patients treated with ICI-based combination therapy, including nivolumab plus ipilimumab, pembrolizumab plus axitinib, or avelumab plus axitinib, as first-line therapy. Patients were classified into the trial-ineligible group when they had at least one of the following factors at the time of treatment initiation: Karnofsky performance status (KPS) < 70%, hemoglobin level < 9.0 g/dL, estimated glomerular filtration rate (eGFR) < 40 mL/min/1.73 m2, platelet count < 100,000/µL, neutrophil count < 1500/µL, non-clear cell histology, or brain metastasis. The remaining patients were classified into the trial-eligible group.
Results
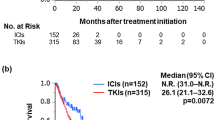
Forty-eight patients (52%) were classified into the trial-ineligible group. The frequency of patients with trial-ineligible factors was highest for low eGFR (n = 20, 45%), followed by non-clear cell histology (n = 17, 36%) and low KPS score (n = 12, 25%). There was no significant difference in progression-free survival (median: 24.0 vs. 11.0 months, p = 0.416), overall survival (1-year rate: 87.0% vs. 85.3%, p = 0.634), or objective response rate (52% vs. 42%, p = 0.308) between the trial-eligible and -ineligible patients. The incidence rate of adverse events was higher in the trial-eligible patients than in the trial-ineligible patients (91% vs. 75%, p = 0.0397); however, the rate of grade 3 or higher adverse events was comparable between the two groups (42% vs. 40%, p = 0.796).
Conclusions
There are many trial-ineligible patients in the real world. Nevertheless, the efficacy and safety of ICI-based combination therapy in trial-ineligible patients were non-inferior compared with those of trial-eligible patients.


Similar content being viewed by others
References
Albiges L, Powles T, Staehler M, Bensalah K, Giles RH, Hora M, et al. Updated European Association of Urology guidelines on renal cell carcinoma: Immune checkpoint inhibition is the new backbone in first-line treatment of metastatic clear-cell renal cell carcinoma. Eur Urol. 2019;76:151–6. https://doi.org/10.1016/j.eururo.2019.05.022.
Motzer RJ, Tannir NM, McDermott DF, Arén Frontera O, Melichar B, Choueiri TK, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378:1277–90. https://doi.org/10.1056/NEJMoa1712126.
Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380:1116–27. https://doi.org/10.1056/NEJMoa1816714.
Motzer RJ, Penkov K, Haanen J, Rini B, Albiges L, Campbell MT, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380:1103–15. https://doi.org/10.1056/NEJMoa1816047.
Kawachi H, Fujimoto D, Morimoto T, Ito M, Teraoka S, Sato Y, et al. Clinical characteristics and prognosis of patients with advanced non-small-cell lung cancer who are ineligible for clinical trials. Clin Lung Cancer. 2018;19:e721–34. https://doi.org/10.1016/j.cllc.2018.05.014.
Sam D, Gresham G, Abdel-Rahman O, Cheung WY. Generalizability of clinical trials of advanced melanoma in the real-world, population-based setting. Med Oncol. 2018;35:110. https://doi.org/10.1007/s12032-018-1167-7.
Lin L, Smit EF, de Langen AJ, van Balen DEM, Beijnen JH, Huitema ADR. Representativeness of Phase III trial for osimertinib in pretreated advanced EGFR-mutated non-small-cell lung cancer patients and treatment outcomes in clinical practice. Target Oncol. 2022;17:53–9. https://doi.org/10.1007/s11523-021-00862-x.
Heng DY, Choueiri TK, Rini BI, Lee J, Yuasa T, Pal SK, et al. Outcomes of patients with metastatic renal cell carcinoma that do not meet eligibility criteria for clinical trials. Ann Oncol. 2014;25:149–54. https://doi.org/10.1093/annonc/mdt492.
Marschner N, Staehler M, Müller L, Nusch A, Harde J, Koska M, et al. Survival of patients with advanced or metastatic renal cell carcinoma in routine practice differs from that in clinical trials-analyses from the German clinical RCC registry. Clin Genitourin Cancer. 2017;15:e209–15. https://doi.org/10.1016/j.clgc.2016.08.022.
Goebell PJ, Staehler M, Müller L, Nusch A, Scheffler M, Sauer A, et al. Changes in treatment reality and survival of patients with advanced clear cell renal cell carcinoma: analyses from the German clinical RCC-registry. Clin Genitourin Cancer. 2018;16:1101-15-e15. https://doi.org/10.1016/j.clgc.2018.06.006.
Ishihara H, Tachibana H, Fukuda H, Yoshida K, Kobayashi H, Takagi T, et al. Prognostic impact of trial-eligibility criteria in patients with metastatic renal cell carcinoma. Urol Int. 2022;106:368–75. https://doi.org/10.1159/000518162.
Gan CL, Stukalin I, Meyers DE, Dudani S, Hai Grosjean DS, et al. Outcomes of patients with solid tumour malignancies treated with first-line immuno-oncology agents who do not meet eligibility criteria for clinical trials. Eur J Cancer. 2021;151:115–25. https://doi.org/10.1016/j.ejca.2021.04.004.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47. https://doi.org/10.1016/j.ejca.2008.10.026.
National Cancer Institute. Common terminology criteria for adverse events (CTCAE), version 40. National Cancer Institute; 2017.
Donia M, Kimper-Karl ML, Høyer KL, Bastholt L, Schmidt H, Svane IM. The majority of patients with metastatic melanoma are not represented in pivotal phase III immunotherapy trials. Eur J Cancer. 2017;74:89–95. https://doi.org/10.1016/j.ejca.2016.12.017.
Batra A, Kong S, Cheung WY. Eligibility of real-world patients with Stage II and III colon cancer for adjuvant chemotherapy trials. Clin Colorectal Cancer. 2020;19:e226–34. https://doi.org/10.1016/j.clcc.2020.05.005.
Yoo SH, Keam B, Kim M, Kim TM, Kim DW, Heo DS. Generalization and representativeness of phase III immune checkpoint blockade trials in non-small cell lung cancer. Thorac Cancer. 2018;9:736–44. https://doi.org/10.1111/1759-7714.12641.
Alhalabi O, Hasanov E, Wilson NR, Araujo J, Wang J, Campbell MT, et al. Outcomes of patients with intermediate-risk or poor-risk metastatic renal cell carcinoma who received their first cycle of nivolumab and ipilimumab in the hospital because of symptomatic disease: the MD Anderson Cancer Center experience. Int J Cancer. 2021;149:387–93. https://doi.org/10.1002/ijc.33560.
Parikh RB, Min EJ, Wileyto EP, Riaz F, Gross CP, Cohen RB, et al. Uptake and survival outcomes following immune checkpoint inhibitor therapy among trial-ineligible patients with advanced solid cancers. JAMA Oncol. 2021;7:1843–50. https://doi.org/10.1001/jamaoncol.2021.4971.
Cella D, Grünwald V, Escudier B, Hammers HJ, George S, Nathan P, et al. Patient-reported outcomes of patients with advanced renal cell carcinoma treated with nivolumab plus ipilimumab versus sunitinib (CheckMate 214): a randomised, phase 3 trial. Lancet Oncol. 2019;20:297–310. https://doi.org/10.1016/S1470-2045(18)30778-2.
Kawashima A, Takayama H, Arai Y, Tanigawa G, Nin M, Kajikawa J, et al. One-month relative dose intensity of not less than 50% predicts favourable progression-free survival in sorafenib therapy for advanced renal cell carcinoma in Japanese patients. Eur J Cancer. 2011;47:1521–6. https://doi.org/10.1016/j.ejca.2011.04.001.
Ishihara H, Takagi T, Kondo T, Iwamoto K, Tachibana H, Yoshida K, et al. Decreased relative dose intensity during the early phase of treatment impacts the therapeutic efficacy of sunitinib in metastatic renal cell carcinoma. Jpn J Clin Oncol. 2018;48:667–72. https://doi.org/10.1093/jjco/hyy078.
Martini DJ, Hamieh L, McKay RR, Harshman LC, Brandao R, Norton CK, et al. Durable clinical benefit in metastatic renal cell carcinoma patients who discontinue PD-1/PD-L1 therapy for immune-related adverse events. Cancer Immunol Res. 2018;6:402–8. https://doi.org/10.1158/2326-6066.CIR-17-0220.
McDermott DF, Drake CG, Sznol M, Choueiri TK, Powderly JD, Smith DC, et al. Survival, durable response, and long-term safety in patients with previously treated advanced renal cell carcinoma receiving nivolumab. J Clin Oncol. 2015;33:2013–20. https://doi.org/10.1200/JCO.2014.58.1041.
Takagi T, Yoshida K, Kobayashi H, Kondo T, Iizuka J, Okumi M, et al. Durable response after discontinuation of nivolumab therapy in patients with metastatic renal cell carcinoma. Jpn J Clin Oncol. 2018;48:860–3. https://doi.org/10.1093/jjco/hyy106.
Ishihara H, Nemoto Y, Nakamura K, Ikeda T, Tachibana H, Fukuda H, et al. Prognostic impact of early treatment interruption of nivolumab plus ipilimumab due to immune-related adverse events as first-line therapy for metastatic renal cell carcinoma: a multi-institution retrospective study. Target Oncol. 2021;16:493–502. https://doi.org/10.1007/s11523-021-00825-2.
Ishihara H, Tachibana H, Takagi T, Yoshida K, Kondo T, Tanabe K. Effect of improved systemic therapy on patient survival in metastatic non-clear-cell renal cell carcinoma. Int J Urol. 2021;28:605–7. https://doi.org/10.1111/iju.14523.
Acknowledgments
The authors thank Ms. Nobuko Hata (Department of Urology, Tokyo Women’s Medical University) for her secretarial work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No external funding was used in the preparation of this article.
Conflicts of interest
Toshio Takagi received honoraria from Bristol-Myers Squibb and Ono Pharmaceutical. Tsunenori Kondo received honoraria from Pfizer, Novartis, Bristol-Myers Squibb, and Ono Pharmaceutical. Yuki Nemoto, Hiroki Ishihara, Kazutaka Nakamura, Hidekazu Tachibana, Hironori Fukuda, Kazuhiko Yoshida, Hirohito Kobayashi, Junpei Iizuka, Hiroaki Shimmura, Yasunobu Hashimoto, and Kazunari Tanabe have no conflicts of interest relevant to the contents of this article.
Ethics approval
The study protocol was approved by the Institutional Ethics Review Board of each institution (Tokyo Women’s Medical University, Tokyo Women’s Medical University Adachi Medical Center, Saiseikai Kawaguchi General Hospital, Saiseikai Kurihashi Hospital, and Jyoban Hospital; ID: 2020-0009). The present study was performed according to the guidelines of the 1964 Declaration of Helsinki and its later amendments. Owing to the retrospective observational nature of this study, the need for informed consent was waived.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Availability of data and materials
Not applicable.
Code availability
Not applicable.
Authors’ contributions
All authors contributed to this study’s conception and design, data collection, and analysis. The first draft of the manuscript was written by Yuki Nemoto and all authors commented on the previous drafts of the manuscript. All authors read and approved the final version of the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nemoto, Y., Ishihara, H., Nakamura, K. et al. Efficacy and Safety of Immunotherapy-Based Combinations as First-Line Therapy for Metastatic Renal Cell Carcinoma in Patients Who Do Not Meet Trial Eligibility Criteria. Targ Oncol 17, 475–482 (2022). https://doi.org/10.1007/s11523-022-00896-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-022-00896-9