Abstract
Background
The MAPK pathway is an emerging target across a number of adult and pediatric tumors. Targeting the downstream effector of MAPK, MEK1, is a proposed strategy to control the growth of MAPK-dependent tumors.
Objective
iMATRIX-cobi assessed the safety, pharmacokinetics, and anti-tumor activity of cobimetinib, a highly selective MEK inhibitor, in children and young adults with relapsed/refractory solid tumors.
Patients and Methods
This multicenter Phase I/II study enrolled patients aged 6 months to < 30 years with solid tumors with known/expected MAPK pathway involvement. Patients received cobimetinib tablet or suspension formulation on Days 1–21 of a 28-day cycle. Dose escalation followed a rolling 6 design. The primary endpoint was safety; secondary endpoints were pharmacokinetics and anti-tumor activity.
Results
Of 56 enrolled patients (median age 9 years [range 3–29]), 18 received cobimetinib tablets and 38 cobimetinib suspension. Most common diagnoses were low-grade glioma (LGG; n = 32, including n = 12 in the expansion cohort) and plexiform neurofibroma within neurofibromatosis type 1 (n = 12). Six patients (11 %) experienced dose-limiting toxicities (including five ocular toxicity events), which established a pediatric recommended Phase II dose (RP2D) of 0.8 mg/kg tablet and 1.0 mg/kg suspension. Most frequently reported treatment-related adverse events were gastrointestinal and skin disorders. Steady state mean exposure (Cmax, AUC0–24) of cobimetinib at the RP2D (1.0 mg/kg suspension) was ~ 50 % lower than in adults receiving the approved 60 mg/day dose. Overall response rate was 5.4 % (3/56; all partial responses in patients with LGG).
Conclusions
The safety profile of cobimetinib in pediatrics was similar to that reported in adults. Clinical activity was observed in LGG patients with known/suspected MAPK pathway activation. Cobimetinib combination regimens may be required to improve response rates in this pediatric population.
Clinical Trial Registration
ClinicalTrials.gov NCT02639546, registered December 24, 2015.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
iMATRIX-cobi is the first study to investigate the safety, pharmacokinetics, and therapeutic activity of cobimetinib in pediatric and young adult patients with previously treated solid tumors. |
The single-agent safety profile of cobimetinib in pediatric patients was similar to that observed in adults, but steady state mean exposure of cobimetinib at the RP2D was approximately 50 % lower than in adults receiving the approved dose. |
A best objective response of partial response was seen in three patients with LGG; combination regimens may further improve response rates in this patient population. |
1 Introduction
Dysregulation of the MAPK pathway is a major oncogenic driver in a number of pediatric tumors [1,2,3,4,5,6,7], most notably low-grade glioma (LGG) [8,9,10]. Targeting MEK1, a downstream effector of MAPK, is a proposed strategy to control the growth of tumors that depend on this signaling cascade.
Cobimetinib is a highly selective inhibitor of MEK1/2. The recommended Phase II dose (RP2D) in adults is 60 mg/day (Days 1−21 of each 28-day cycle), with most frequent toxicities of diarrhea, rash, fatigue, and edema [11]. Cobimetinib has a moderate rate of absorption in adults, with a median time to maximum concentration of 2.4 hours [12]. Combining cobimetinib and the BRAF inhibitor vemurafenib significantly improved investigator-assessed progression-free survival (PFS) and objective response rate (ORR; both p < 0.0001) versus vemurafenib plus placebo in a randomized Phase III study in adults with unresectable/metastatic BRAF V600E/K-mutated melanoma [12]. These data led to the approval of cobimetinib for the treatment of adults in this setting in combination with vemurafenib [13, 14].
Pediatric data for cobimetinib are lacking. Cytotoxic effects have been demonstrated on a number of pediatric cancer cell lines, with activity observed in xenograft models of pediatric solid tumors [Roche data on file]. We report results from a Phase I/II study evaluating the safety, tolerability, and pharmacokinetics of cobimetinib, as well as preliminary anti-tumor activity and biomarker data, in pediatric and young adult patients with relapsed/refractory solid tumors.
2 Materials and Methods
2.1 Study Design and Participants
iMATRIX-cobi (NCT02639546; ITCC-055) was a multicenter, open-label Phase I/II study. Patients were recruited from seven countries as part of the European Innovative Therapies for Children with Cancer Consortium and Pediatric Oncology Experimental Therapeutics Investigators’ Consortium. Enrollment occurred in two stages: dose escalation (tablet and suspension formulations) and expansion at the RP2D for patients with LGG. Patients were aged ≥ 6 years and < 18 years for the tablet, ≥ 6 months and <18 years for the suspension, and ≥ 6 months and < 30 years for expansion.
Eligible patients had histologically/cytologically confirmed tumors with known/expected MAPK pathway involvement. These included: gliomas (high-grade glioma [HGG] and LGG); embryonal rhabdomyosarcoma (RMS) and non-RMS soft tissue sarcomas; neuroblastoma; melanoma; malignant peripheral nerve sheath tumor; rhabdoid tumors, including atypical teratoid/rhabdoid tumor; neurofibromatosis-1 (NF-1)–associated tumors (including plexiform neurofibroma [PN]) and schwannoma judged by the investigator to be life-threatening, resulting in severe symptoms, or in close proximity to vital structures; and any solid tumor or brain tumor that occurs with another RASopathy, such as Noonan syndrome. Patients had measurable/evaluable disease according to International Neuroblastoma Response Criteria (INRC) [15], Response Assessment in Neuro-Oncology (RANO) for HGG [16], or Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 [17]; availability of tumor tissue; Lansky or Karnofsky performance status ≥ 50 % for children aged < 16 or ≥ 16 years, respectively; and life expectancy ≥ 3 months. Prior to the availability of the suspension, body weight had to be ≥ 20 kg.
Exclusion criteria included: prior MEK inhibitors; high-dose chemotherapy or stem-cell rescue within 3 months; chemotherapy, differentiation therapy, or immunotherapy within 4 weeks; thoracic or mediastinal radiotherapy within 6 weeks; biologic or herbal cancer therapy within 4 weeks or < 5 half-lives; investigational therapy within 4 weeks; inability to swallow oral medication; and impaired gastrointestinal absorption.
The study protocol was approved by the medical authorities, institutional review board, and ethics committees according to local legislations and complied with Good Clinical Practice guidelines and the principles of the Declaration of Helsinki. Written informed consent was obtained from parents, patients, or legal representatives.
2.2 Procedures
Two pediatric formulations of cobimetinib were used: suspension or 20-mg tablets, taken orally, Days 1–21 of each 28-day cycle, with/without food. Patients received a cumulative weekly dose (rounded to the nearest 20 mg) based on body weight.
Dose escalation investigated 0.6, 0.8, and 1.0 mg/kg tablets, and 0.6, 0.8, 1.0, and 1.33 mg/kg suspension (doses were capped at the adult 60 mg/day dose). Cohorts of 3–6 patients were treated at escalating doses according to the rolling 6 design. Dose-limiting toxicity (DLT) was assessed during cycle 1 (see electronic supplementary material [ESM]).
During dose expansion, patients with LGG with documented MAPK pathway activation were enrolled. The primary analysis was conducted after enrollment was completed and all enrolled patients had been followed for at least 12 months. Patients received cobimetinib as long as clinical benefit was perceived, or until disease progression (PD), death, unacceptable toxicity, or decision to discontinue.
2.3 Outcomes
The primary objective was safety and tolerability of cobimetinib. Secondary objectives: characterization of cobimetinib pharmacokinetics; anti-tumor activity (ORR and PFS); duration of response (DoR); and overall survival (OS). Exploratory objectives: analysis of MAPK pathway biomarkers in tumor tissue at baseline, on treatment, and at PD.
2.4 Assessments
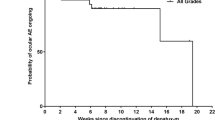
Adverse events (AEs) were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events v4.0. Patients underwent ophthalmologic examinations including spectral domain optical coherence tomography (OCT) at baseline, Days 8 and 15 of cycle 1, Day 1 of each subsequent cycle, and study drug discontinuation. Plasma samples were collected pre-dose, at 2-, 4-, 6-, and 24-hours post-dose on Days 1 and 21 of cycle 1, and within 4 h pre-dose on Day 1 of cycle 2. Cobimetinib plasma concentrations were measured using validated liquid chromatography–tandem mass spectroscopy (Covance, Madison, WI, USA). Estimated pharmacokinetic parameters were maximum plasma concentration (Cmax) and exposure (area under the concentration-time curve from 0 to 24 hours [AUC0–24]). Tumor assessments were performed after every 2 cycles. Investigator-assessed response was determined using INRC, RANO, or RECIST v1.1. In the expansion phase, response was assessed both by RANO and RECIST. Objective response rate was defined as a complete response (CR) or partial response (PR) on two consecutive occasions ≥ 4 weeks apart. Responses were assessed locally, with no central response assessment performed. Progression-free survival was the time from initiation of cobimetinib to occurrence of PD or death, whichever occurred first. Duration of response was the time from the first tumor assessment to the time of PD or death. Overall survival was the time from initiation of cobimetinib to death.
2.5 Statistical Analysis
Safety, pharmacokinetic, and therapeutic activity were assessed within the safety-evaluable population, comprising all patients who received study drug. Cobimetinib plasma concentrations were used to perform a non-compartmental pharmacokinetic analysis with Phoenix WinNonlin (v6.4, Certera, Princeton, NJ, USA). Median time-to-event endpoints were estimated by means of the Kaplan-Meier approach, with 95 % confidence intervals (CI) estimated according to Brookmeyer and Crowley [18]. The clinical cut-off date was November 27, 2019. All patients were followed-up for ≥ 12 months.
3 Results
3.1 Patient Demographics and Characteristics
Between May 2016 and November 2018, 56 patients were enrolled and treated. Most common tumor types were LGG (n = 32, including three NF1-related LGG), NF1-associated PN (n = 12), and HGG (n = 5) (Table 1). Eighteen patients received cobimetinib tablets (0.6–1.0 mg/kg) and 26 the suspension (0.6–1.33 mg/kg). Twelve LGG patients received the suspension (1.0 mg/kg) during dose expansion. All patients were evaluable for safety, pharmacokinetics, and anti-tumor activity.
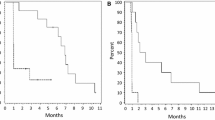
Median patient age was 9 years (range 3–29). Patients were enrolled at a median of 39.1 months post-diagnosis (range 1.5–153.5), following a median of 5.0 prior treatment lines (range 0−18). At clinical cut-off, 12 patients (21 %) remained on treatment, and 19 (34 %) were in follow-up (Fig. 1). Median cobimetinib exposure was 4.1 months (range 0.3–41.2).
3.2 Safety and Tolerability
Six patients (11 %) had DLTs: 1.0 mg/kg tablet (grade 3 headache, grade 2 retinal detachment); 0.6 mg/kg suspension (grade 1 chorioretinopathy); 1.33 mg/kg suspension (grade 4 chorioretinopathy, grade 1 serous retinal detachment, grade 1 pigment epithelial detachment) (ESM, Table S1). The majority of these were resolving/had resolved. Thus, the pediatric RP2D was 0.8 mg/kg tablet and 1.0 mg/kg suspension.
The AE profile was comparable across formulations and age groups (ESM, Table S2). A similar proportion of patients experienced treatment-related AEs (TRAEs) during dose escalation (89 % for both formulations) and expansion (92 %); most were grade 1/2 (55 %) (Table 2). Grade 3/4 AEs occurred at a comparable rate during dose escalation (50 % tablet, 39 % suspension) and expansion (50 %). There were no grade 5 AEs.
Adverse events of special interest (AESI) occurred in 25 patients (45 %): 33 % during tablet dose escalation, 50 % during suspension dose escalation, and 50 % during expansion. Most common AESIs were epistaxis (dose escalation: 17 % tablet, 12 % suspension; expansion: 33 %), aspartate aminotransferase increased (dose escalation: 6 % tablet, 12 % suspension; expansion: 17 %), and creatine phosphokinase increased (expansion: 17 %).
Six patients (11 %) experienced TRAEs leading to cobimetinib withdrawal: 6 % during tablet dose escalation (1.0 mg/kg: grade 2 retinal detachment), 15 % during suspension dose escalation (1.0 mg/kg: grade 2 epitheliopathy/keratitis; 1.33 mg/kg: grade 4 chorioretinopathy, grade 2 ECG QT prolonged, grade 1 serous retinal detachment), and 8 % during expansion (grade 4 encephalopathy). Five of these were DLTs. Eleven deaths (20 %) occurred on study, all due to PD.
3.3 Pharmacokinetics
Figure 2 shows mean plasma concentration-time profiles following single- (n = 26) and multiple-dose (n = 22) administration of cobimetinib suspension. Steady state mean cobimetinib exposure (Cmax, AUC0–24) at the pediatric RP2D in the expansion cohort (142 ng/mL and 1862 ng*h/mL, respectively) was ~ 50 % lower than exposures reported for adults receiving the approved dose (mean steady state Cmax and AUC0–24h: 273 ng/mL and 4340 ng*h/mL, respectively [12]), but high interpatient variability was observed. Mean steady state exposure (AUC0–24) in pediatric patients was 2.5-fold higher than with single-dose exposure, consistent with the mean accumulation ratio in adults (2.4-fold) [12].
Mean steady state cobimetinib exposure for 1.0 mg/kg suspension during expansion (n = 9) was lower than during dose escalation (n = 8). The geometric mean (%CV) for Cmax was 116 (42 %) versus 179 (113 %) ng/mL and for AUC0–24 was 1402 (59 %) versus 2562 (104 %) ng*h/mL. However, there was overlap in individual exposures between the cohorts (ESM, Fig. S1). Although the half-life of cobimetinib could not be estimated, it was expected to be similar to the half-life of 44 hours observed in adults [12].
3.4 Anti-Tumor Activity
No CRs were reported. Overall, 3/56 patients (5 %) had a PR (all with LGG) and 33/56 (59 %) had stable disease (median 11.0 months) (Table 3). Tumors from two responding patients were reported to have MAPK pathway alterations, including an NF1 mutation and an unspecified mutation (ESM, Table S3). Disease progression occurred in 23 % of patients, 2 % had non-CR/non-PD, 4 % were non-evaluable, and data were unavailable for 7 %.
Among the expansion cohort, 1/12 LGG patients (8 %) achieved a PR and 1/12 (8 %) had a minor response (Table 3; ESM, Fig. 2). Stable disease was recorded in 8/12 patients (67 %) (median 7.2 months). Median DoR was not reached.
Median duration of survival follow-up was 15.0 months (range 0.7–41.6). Median PFS was 20.2 months (95 % CI 9.3−not estimable) for all LGG patients and 14.8 months (95 % CI 3.6−14.8) for the expansion cohort (Fig. 3). Median OS in the overall population was not reached.
3.5 MAPK Pathway Biomarkers
Local molecular data were provided for 36 patients. Among 28 LGG patients, 23 tumors were reported to have MAPK pathway alterations: 18 harbored a BRAF alteration (primarily KIAA1549-BRAF fusions or other BRAF duplication), one an NF1 mutation, and four had unspecified mutations (ESM, Table S4). According to available information, five tumors had no pathway mutation, and no molecular data were provided for four patients.
pERK1/2 scores were available for three patients, two of whom also had pMEK1/2 scores (ESM, Fig. 3a, b). Analysis of pERK1/2/pMEK1/2 inhibition was therefore not feasible. Ki67 scores were available for 33 patients (ESM, Fig. 3c).
4 Discussion
iMATRIX-cobi is the first study to investigate cobimetinib in pediatric and young adult patients with previously treated solid tumors. Cobimetinib was generally tolerable, with a safety profile consistent with that reported in adults. Most AEs were grade 1/2.
The pediatric RP2D of cobimetinib was determined to be 0.8 mg/kg tablet and 1.0 mg/kg suspension. Steady state mean exposure (Cmax, AUC0–24) in patients receiving 1.0 mg/kg cobimetinib suspension was ~ 50 % lower than in adults receiving the approved dose [12]. Since higher doses were not tolerated, it was not possible to dose pediatric patients to match the adult exposure. One hypothesis for the lower exposure is differences in CYP3A variations in gut/liver in young children versus adults. In adults, cobimetinib AUC was 50 % lower in healthy subjects versus cancer patients at a similar dose. Exposures in pediatric patients with LGG are comparable to those in healthy subjects, indicating similarity with respect to cobimetinib disposition [19]. There were no exposure-safety relationships in pediatric patients, similar to adults [20].
Precision oncology trials have investigated MEK inhibition in relapsed/refractory pediatric tumors [reviewed in 21]. The ORR we observed was low, with a PR of 5 %, all in LGG patients. Some patients (59 %), particularly with LGG and NF1-associated PN, experienced prolonged stable disease, though PFS was not the primary study endpoint.
Phase I investigation of the MEK inhibitor selumetinib in pediatric patients with recurrent/refractory LGG, 58 % with pilocytic astrocytoma, revealed a PR (based on bidimensional measurements on T2/FLAIR) of 20 % (5/25; four with BRAF aberrations) and a 2-year PFS of 69 % [22]. Partial response rates of 36 % (9/25) and 40 % (10/25), respectively, were reported with selumetinib in a Phase II study in children with BRAF aberrations (KIAA1549-BRAF fusion or BRAF V600E mutation) or NF1-type LGG [23]. A considerably higher PR rate of 74 % (68 % confirmed) was reported in a Phase II selumetinib study in pediatric patients with NF1 and inoperable PN, with stable disease in 12 % [24]. This suggests that sensitivity to MEK inhibition may depend on the molecular alteration within the MAPK pathway.
Final results from the trametinib study in pediatric patients with NF1-associated PN are awaited. Preliminary data reported 12/26 patients (46 %) with PR and 10/12 responses (83 %) ongoing [25]. The selumetinib and trametinib studies employed Dombi’s criteria [26], whereas our study used RECIST for NF1-associated PN. These differences may in part explain the observed lower ORR.
In pediatric LGG, aberrant MAPK signaling commonly occurs through BRAF activation, often involving a KIAA1549-BRAF gene fusion or an activating BRAF V600E point mutation [21]. Of our 28 LGG patients with available molecular data, 18 had tumors that harbored a BRAF alteration (primarily KIAA1549-BRAF fusion), one had an NF1 mutation, and four had unspecified mutations. Thus, in LGG patients with site-reported pathway aberrations, the ORR was 13 % (3/23). While differences in tumor assessment criteria may have played a role, the ORR for cobimetinib was not as high as reported with other MEK inhibitors.
The lack of prospective determination of NF1/RAS/RAF/MEK/ERK alterations, and/or central confirmation of local molecular data, is a major limitation of our study. Selection of patients with tumors harboring known MAPK pathway alterations may have yielded a higher ORR or enabled a better understanding of responses against pathway activation status. In common with other MEK inhibitors [27], cobimetinib can act as a substrate of P-glycoprotein [28]; therefore, cobimetinib may not have reached sufficient concentration for optimal therapeutic benefit.
The DLT criteria and the occurrence of ocular toxicities might have contributed to the relatively low exposure of cobimetinib. While the DLT criteria defined in this study with respect to ocular toxicities were very stringent, such a conservative approach was essential for the population being studied. Seven patients had an optic pathway LGG, and symptomatic retinal detachment would have had potentially serious consequences for those whose vision was already compromised. Future studies would need to assess the benefit-risk ratio based on the specific indication to determine whether such DLT criteria were appropriate. The relevance of ocular DLTs being asymptomatic remains unclear, but may have been because they were detected early, with OCT conducted on Day 8, cycle 1. The early and more frequent ocular testing implemented in this study was primarily due to the known ocular toxicities observed in adults, which mostly occurred around cycle 1 or 2 of treatment.
5 Conclusions
Cobimetinib was well tolerated in pediatric and young adult patients with refractory/relapsed solid tumors with a pediatric RP2D identified as 0.8 mg/kg tablet and 1.0 mg/kg suspension. With only a few responses, the criteria to proceed to further cohort expansion were not met. Combination regimens and stringent selection for molecular alteration may hold more promise for optimizing therapeutic outcomes for pediatric patients with solid tumors.
References
Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–310.
Forshew T, Tatevossian RG, Lawson AR, Ma J, Neale G, Ogunkolade BW, et al. Activation of the ERK/MAPK pathway: a signature genetic defect in posterior fossa pilocytic astrocytomas. J Pathol. 2009;218:172–81.
Sievert AJ, Lang SS, Boucher KL, Madsen PJ, Slaunwhite E, Choudhari N, et al. Paradoxical activation and RAF inhibitor resistance of BRAF protein kinase fusions characterizing pediatric astrocytomas. Proc Natl Acad Sci USA. 2013;110:5957–62.
Vitagliano O, Addeo R, D’Angelo V, Indolfi C, Indolfi P, Casale F. The Bcl-2/Bax and Ras/Raf/MEK/ERK signaling pathways: implications in pediatric leukemia pathogenesis and new prospects for therapeutic approaches. Expert Rev Hematol. 2013;6:587–97.
Shern JF, Chen L, Chmielecki J, Wei JS, Patidar R, Rosenberg M, et al. Comprehensive genomic analysis of rhabdomyosarcoma reveals a landscape of alterations affecting a common genetic axis in fusion-positive and fusion-negative tumors. Cancer Discov. 2014;4:216–31.
Knight T, Irving JAE. Ras/Raf/MEK/ERK pathway activation in childhood acute lymphoblastic leukemia and its therapeutic targeting. Front Oncol. 2014;4:160.
Xie G, Wu H, Cai W, Chen M, Huang W, Yan W, et al. RDM1 promotes neuroblastoma growth through the RAS–Raf–MEK–ERK pathway. FEBS Open Bio. 2019;9:490–7.
Packer RJ, Pfister S, Bouffet E, Avery R, Bandopadhayay P, Bornhorst M, et al. Pediatric low-grade gliomas: Implications of the biologic era. Neuro Oncol. 2017;19:750–61.
Tateishi K, Nakamura T, Yamamoto T. Molecular genetics and therapeutic targets of pediatric low-grade gliomas. Brain Tumor Pathol. 2019;36:74–83.
de Blank P, Bandopadhayay P, Haas-Kogan D, Fouladi M, Fangusaro J. Management of pediatric low-grade glioma. Curr Opin Pediatr. 2019;31:21–7.
Rosen LS, LoRusso P, Wee Ma W, Goldman JW, Weise A, Colevas AD, et al. A first-in-human phase I study to evaluate the MEK1/2 inhibitor, cobimetinib, administered daily in patients with advanced solid tumors. Invest New Drugs. 2016;34:604–13.
Cobimetinib SmPC. https://www.ema.europa.eu/en/documents/product-information/cotellic-epar-product-information_en.pdf. Accessed 1 Oct 2021.
FDA. Cotellic PI. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/206192s000lbl.pdf. Accessed 1 Oct 2021.
EMA. Cotellic EPAR summary. https://www.ema.europa.eu/en/medicines/human/EPAR/cotellic. Accessed 1 Oct 2021.
Park JR, Bagatell R, Cohn SL, Pearson AD, Villablanca JG, Berthold F, et al. Revisions to the International Neuroblastoma Response Criteria: a consensus statement from the National Cancer Institute Clinical Trials planning meeting. J Clin Oncol. 2017;35:2580–7.
Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2012;28:1963–72.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumors: revised RECIST guideline (Version 1.1). Eur J Cancer. 2009;45:228–47.
Brookmeyer R, Crowley J. A confidence interval for the median survival time. Biometrics. 1982;38:29–41.
Budha NR, Ji T, Musib L, Eppler S, Dresser M, Chen Y, et al. Evaluation of cytochrome P450 3A4-mediated drug–drug interaction potential for cobimetinib using physiologically based pharmacokinetic modeling and simulation. Clin Pharmacokinet. 2016;55:1435–45.
Center for Drug Evaluation and Research. Clinical Pharmacology and Biopharmaceutics Filing for Cobimetinib. 2015. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/206192Orig1s000ClinPharmR.pdf. Accessed 1 Oct 2021.
Forrest SJ, Geoerger B, Janeway KA. Precision medicine in pediatric oncology. Curr Opin Pediatr. 2018;30:17–24.
Banerjee A, Jakacki RI, Onar-Thomas A, Wu S, Nicolaides T, Poussaint TY, et al. A phase I trial of the MEK inhibitor selumetinib (AZD6244) in pediatric patients with recurrent or refractory low-grade glioma: A Pediatric Brain Tumor Consortium (PBTC) study. Neuro Oncol. 2017;19:1135–44.
Fangusaro J, Onar-Thomas A, Poussaint TY, Wu S, Ligon AH, Lindeman N, et al. Selumetinib in paediatric patients with BRAF-aberrant or neurofibromatosis type 1-associated recurrent, refractory, or progressive low-grade glioma: a multicentre, phase 2 trial. Lancet Oncol. 2019;20:1011–22.
Gross AM, Wolters PL, Dombi E, Baldwin A, Whitcomb P, Fisher MJ, et al. NFM-07. Selumetinib in children with inoperable plexiform neurofibromas. N Engl J Med. 2020;382:1430–42.
McCowage GB, Mueller S, Pratilas CA, Hargrave DR, Moertel CL, Whitlock J, et al. Trametinib in pediatric patients with neurofibromatosis type 1 (NF-1)–associated plexiform neurofibroma: a phase I/IIa study. J Clin Oncol. 2018;36(15_Suppl):10504.
Dombi E, Baldwin A, Marcus LJ, Fisher MJ, Weiss B, Kim AR, et al. Activity of selumetinib in neurofibromatosis type 1-related plexiform neurofibromas. N Engl J Med. 2016;375:2550–60.
de Gooijer MC, Zhang P, Weijer R, Buil LCM, Beijnen JH, van Tellingen O. The impact of P-glycoprotein and breast cancer resistance protein on the brain pharmacokinetics and pharmacodynamics of a panel of MEK inhibitors. Int J Cancer. 2018;142:381–91.
Choo EF, Ly J, Chan J, Shahidi-Latham SK, Messick K, Plise E, et al. Role of P-glycoprotein on the brain penetration and brain pharmacodynamic activity of the MEK inhibitor cobimetinib. Mol Pharm. 2014;11:4199–207.
Acknowledgements
The authors thank the patients, their families, the participating study centers and the European Innovative Therapies for Children with Cancer Consortium and Pediatric Oncology Experimental Therapeutics Investigators’ Consortium. They also acknowledge the help of Stephen Simko (Senior Medical Director, Genentech, Inc.), Hubert Caron (Group Medical Director, F. Hoffmann-La Roche Ltd), Cecile Guizani (Roche Clinical Science) and Silver Alkhafaji (Genentech Clinical Pharmacology). Third-party medical writing assistance, under the direction of the authors, was provided by Fiona Fernando, PhD, contract medical writer at Ashfield MedComms, an Ashfield Health company, and was funded by F. Hoffmann-La Roche Ltd.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The study was funded by F. Hoffmann-La Roche Ltd/Genentech, Inc.
Conflicts of interest
TT reports consultancy fees from F. Hoffmann-La Roche Ltd. HT reports lecture fees from AstraZeneca and Roche. SG reports personal fees from Bayer, EUSA Pharma, and LOXO Oncology. CR reports personal fees from Amgen, BMS, Celgene, Genentech, Inc., Novartis, Pfizer, and F. Hoffmann-La Roche Ltd. DP was an employee of F. Hoffman-La Roche Ltd. at the time of study conduct. LM was an employee of Genentech, Inc. at the time of study conduct. LRP is an employee of F. Hoffmann-La Roche Ltd. SC, KEH, CD, and RB are employees of and stock-holders in F. Hoffmann-La Roche Ltd/Genentech, Inc. All other authors declare no competing interests.
Ethics approval
This study was performed in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent to participate
Written informed consent was obtained from parents, patients, or legal representatives.
Consent for publication
Not applicable.
Data sharing
Qualified researchers may request access to individual patient level data through the clinical study data request platform (https://vivli.org/). Further details on Roche's criteria for eligible studies are available here (https://vivli.org/members/ourmembers/). For further details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here (https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm).
Code availability
Not applicable.
Author contributions
TT and BG contributed to the conception and design of the study. All authors contributed to collection and assembly of data, and to data analysis and interpretation. All authors critically reviewed the manuscript, provided final approval, and agreed to be accountable for all aspects of the work.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Trippett, T., Toledano, H., Campbell Hewson, Q. et al. Cobimetinib in Pediatric and Young Adult Patients with Relapsed or Refractory Solid Tumors (iMATRIX-cobi): A Multicenter, Phase I/II Study. Targ Oncol 17, 283–293 (2022). https://doi.org/10.1007/s11523-022-00888-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-022-00888-9