Abstract
Background
The efficacy of crizotinib for anaplastic lymphoma kinase (ALK)-rearranged non-small-cell lung cancer (NSCLC) patients with brain metastasis is controversial. Real-world research data are needed as further evidence.
Objective
We conducted a multicenter, retrospective study to explore how crizotinib affects the control of brain metastasis and the survival outcomes among Chinese patients.
Patients and Methods
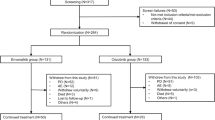
We reviewed the medical records of unselected ALK-rearranged NSCLC patients treated with crizotinib at five hospitals in China from January 1, 2013 to November 30, 2017. Patients developing brain metastasis either before or during crizotinib treatment were included. Survival outcomes were analyzed by the Kaplan–Meier method, and prognostic factors were analyzed by multivariate Cox regression.
Results
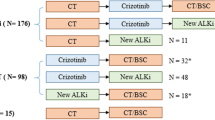
A total of 174 patients were included in the analysis; 95 of these patients had baseline brain metastasis, while 79 patients developed brain metastasis during crizotinib treatment. Among patients with baseline brain metastasis, the median intracranial time to progression was 19.3 months (95% confidence interval [CI] 12.5–26.2) and the median overall survival (OS) was 53.4 months (95% CI not reached). A total of 135 patients experienced intracranial progression, and 94 of these patients continued crizotinib beyond progressive disease (CBPD). There was no significant difference in the median OS between patients with CBPD and without CBPD (48.3 months vs 53.4 months; p = 0.296).
Conclusions
ALK-rearranged advanced NSCLC patients with baseline brain metastasis can still achieve OS benefits from crizotinib treatment. However, patients with intracranial progression may not obtain a long-term survival benefit from continuation of CBPD.




Similar content being viewed by others
References
The National Comprehensive Cancer Network (NCCN): Clinical Practice Guidelines in Oncology: non-small cell lung cancer, 2018; version 3. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp-nscl.
Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. New Engl J Med. 2010;363(18):1693–703. https://doi.org/10.1056/NEJMoa1006448.
Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol. 2009;27(26):4247–53. https://doi.org/10.1200/jco.2009.22.6993.
Sun JM, Lira M, Pandya K, et al. Clinical characteristics associated with ALK rearrangements in never-smokers with pulmonary adenocarcinoma. Lung Cancer (Amsterdam, Netherlands). 2014;83(2):259–64. https://doi.org/10.1016/j.lungcan.2013.11.009.
Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. New Engl J Med. 2014;371(23):2167–77. https://doi.org/10.1056/NEJMoa1408440.
Solomon BJ, Kim DW, Wu YL, et al. Final overall survival analysis from a study comparing first-line crizotinib versus chemotherapy in ALK-mutation-positive non-small-cell lung cancer. J Clin Oncol. 2018;36(22):2251–8. https://doi.org/10.1200/jco.2017.77.4794.
Wu YL, Lu S, Lu Y, et al. Results of PROFILE 1029, a phase III comparison of first-line crizotinib versus chemotherapy in East Asian patients with ALK-positive advanced non-small cell lung cancer. J Thor Oncol. 2018;13(10):1539–48. https://doi.org/10.1016/j.jtho.2018.06.012.
Dagogo-Jack I, Gill CM, Cahill DP, Santagata S, Brastianos PK. Treatment of brain metastases in the modern genomic era. Pharmacol Ther. 2017;170:64–72. https://doi.org/10.1016/j.pharmthera.2016.10.011.
Costa DB, Shaw AT, Ou SH, et al. Clinical experience with crizotinib in patients with advanced ALK-rearranged non-small-cell lung cancer and brain metastases. J Clin Oncol. 2015;33(17):1881–8. https://doi.org/10.1200/jco.2014.59.0539.
Wardak Z, Choy H. Improving treatment options for brain metastases from ALK-positive non-small-cell lung cancer. J Clin Oncol. 2016;34(34):4064–5. https://doi.org/10.1200/jco.2016.69.9587.
Rusthoven CG, Doebele RC. Management of brain metastases in ALK-positive non-small-cell lung cancer. J Clin Oncol. 2016;34(24):2814–9. https://doi.org/10.1200/jco.2016.67.2410.
Costa DB, Kobayashi S, Pandya SS, et al. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol. 2011;29(15):e443–5. https://doi.org/10.1200/jco.2010.34.1313.
Camidge DR, Peters S, Mok T, et al. Updated efficacy and safety data from the global phase III ALEX study of alectinib (ALC) vs crizotinib (CZ) in untreated advanced ALK + NSCLC. J Clin Oncol. 2018;36(suppl): abstr 9043.
Gadgeel S, Peters S, Mok T, et al. Alectinib versus crizotinib in treatment-naive anaplastic lymphoma kinase-positive (ALK +) non-small-cell lung cancer: CNS efficacy results from the ALEX study. Ann Oncol. 2018;29(11):2214–22. https://doi.org/10.1093/annonc/mdy405.
Camidge DR, Kim HR, Ahn MJ, et al. Brigatinib versus crizotinib in ALK-positive non-small-cell lung cancer. New Engl J Med. 2018;379(21):2027–39. https://doi.org/10.1056/NEJMoa1810171.
Solomon BJ, Cappuzzo F, Felip E, et al. Intracranial efficacy of crizotinib versus chemotherapy in patients with advanced ALK-positive non-small-cell lung cancer: results from PROFILE 1014. J Clin Oncol. 2016;34(24):2858–65. https://doi.org/10.1200/jco.2015.63.5888.
Pacheco JM, Gao D, Smith D, et al. Natural history and factors associated with overall survival in stage IV ALK-rearranged non-small cell lung cancer. J Thorac Oncol. 2019;14(4):691–700. https://doi.org/10.1016/j.jtho.2018.12.014.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The study was funded by Pfizer investigator funding.
Conflict of interest
Junling Li has recived Pfizer investigator funding. Puyuan Xing, Shouzheng Wang, Qiang Wang, Di Ma, Xuezhi Hao, Mengzhao Wang, Yan Wang, Li Shan, Tao Xin, Li Liang, Hongge Liang, Yang Du, and Zhaohui Zhang declare that they have no conflicts of interest that might be relevant to the contents of this manuscript.
Rights and permissions
About this article
Cite this article
Xing, P., Wang, S., Wang, Q. et al. Efficacy of Crizotinib for Advanced ALK-Rearranged Non-Small-Cell Lung Cancer Patients with Brain Metastasis: A Multicenter, Retrospective Study in China. Targ Oncol 14, 325–333 (2019). https://doi.org/10.1007/s11523-019-00637-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-019-00637-5