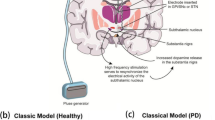
Abstract
Traditional deep brain stimulation (DBS) is one of the acceptable methods to relieve the clinical symptoms of Parkinson’s disease in its advanced stages. Today, the use of closed-loop DBS to increase stimulation efficiency and patient satisfaction is one of the most important issues under investigation. The present study was aimed to find local field potential (LFP) features of parkinsonian rats, which can determine the timing of stimulation with high accuracy. The LFP signals from rats were recorded in three groups of parkinsonian rat models receiving stimulation (stimulation), without getting stimulation (off-stimulation), and sham-controlled group. The frequency domain and chaotic features of signals were extracted for classifying three classes by support vector machine (SVM) and neural networks. The best combination of features was selected using the genetic algorithm (GA). Finally, the effective features were introduced to determine the on/off stimulation time, and the optimal stimulation parameters were identified. It was found that a combination of frequency domain and chaotic features with an accuracy of about 99% was able to determine the time the DBS must switch on. In about 80.67% of the 1861 different stimulation parameters, the brain was able to maintain its state for about 3 min after stimulation discontinuation.
Graphical abstract







Similar content being viewed by others
References
Zhao D, Sun Q, Cheng S, He M, Chen X, Hou X (2018) Extraction of Parkinson’s disease-related features from local field potentials for adaptive deep brain stimulation. Neurophysiology 50:57–67
Little S, Brown P (2012) What brain signals are suitable for feedback control? Ann N Y Acad Sci 1265:9–24
Groiss SJ, Wojtecki L, Südmeyer M, Schnitzler A (2009) Deep brain stimulation in Parkinson’s disease. Ther Adv Neurol Disord 2:379–391
Amboni M, Santangelo G, Barone P (2015) Depression, apathy, anhedonia, and fatigue in Parkinson’s disease. Neuropsychiatr Symptoms Neurol Disease 1–28
Krack P, Batir A, Van Blercom N, Chabardes S, Fraix V, Ardouin C, Koudsie A, Limousin PD, Benazzouz A, LeBas JF, Benabid AL (2003) Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson’s disease. N Engl J Med 349(20):1925–34
Schüpbach WM, Chastan N, Welter ML, Houeto JL, Mesnage V, Bonnet AM, Czernecki V, Maltete D, Hartmann A, Mallet L, Pidoux B (2005) Stimulation of the subthalamic nucleus in Parkinson’s disease: a 5 year follow up. J Neurol Neurosurg Psychiatry 76(12):1640–4
Weaver FM, Follett K, Stern M, Hur K, Harris C, Marks WJ, Rothlind J, Sagher O, Reda D, Moy CS, Pahwa R (2009) Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. Jama 301(1):63–73
Wagle Shukla A, Zeilman P, Fernandez H, Bajwa JA, Mehanna R (2017) DBS programming: an evolving approach for patients with Parkinson’s disease. Parkinson’s Disease 1-11
Hell F, Palleis C, Mehrkens JH, Koeglsperger T, Bötzel K (2019) Deep brain stimulation programming 2.0: future perspectives for target identification and adaptive closed loop stimulation. Front Neurol 10
Pan S, Iplikci S, Warwick K, Aziz TZ (2012) Parkinson’s disease tremor classification–a comparison between support vector machines and neural networks. Expert Syst Applic 39(12):10764–71
Ghasemi P, Sahraee T, Mohammadi A (2018) Closed-and open-loop deep brain stimulation: methods, challenges, current and future aspects. J Biomed Phys Eng 8(2):209
Lozano AM, Lipsman N, Bergman H, Brown P, Chabardes S, Chang JW, Matthews K, McIntyre CC, Schlaepfer TE, Schulder M, Temel Y (2019) Deep brain stimulation: current challenges and future directions. Nat Rev Neurol 15(3):148–60
Parastarfeizabadi M, Kouzani AZ (2017) Advances in closed-loop deep brain stimulation devices. J Neuroeng Rehab 14(1):79
Wiecki TV, Poland J, Frank MJ (2015) Model-based cognitive neuroscience approaches to computational psychiatry: clustering and classification. Clin Psychol Sci 3(3):378–99
Liu C, Wang J, Deng B, Wei XL, Yu HT, Li HY (2015) Variable universe fuzzy closed-loop control of tremor predominant Parkinsonian state based on parameter estimation. Neurocomputing 151:1507–18
Mohammed A, Zamani M, Bayford R, Demosthenous A (2017) Toward on-demand deep brain stimulation using online Parkinson’s disease prediction driven by dynamic detection. IEEE Trans Neural Syst Rehab Eng 25(12):2441–52
Ramirez-Zamora A, Giordano JJ, Gunduz A, Brown P, Sanchez JC, Foote KD, Almeida L, Starr PA, Bronte-Stewart HM, Hu W, McIntyre C (2018) Evolving applications, technological challenges and future opportunities in neuromodulation: proceedings of the fifth annual deep brain stimulation think tank. Front Neurosci 11:734
Priori A, Foffani G, Rossi L, Marceglia S (2013) Adaptive deep brain stimulation (aDBS) controlled by local field potential oscillations. Exp Neurol 245:77–86
Friston KJ, Bastos AM, Pinotsis D, Litvak V (2015) LFP and oscillations—what do they tell us? Curr Opin Neurobiol 31:1–6
Habets JG, Heijmans M, Kuijf ML, Janssen ML, Temel Y, Kubben PL (2018) An update on adaptive deep brain stimulation in Parkinson’s disease. Mov Disord 33(12):1834–43
Chen Y et al (2019) Automatic Sleep Stage Classification Based on Subthalamic Local Field Potentials. IEEE Trans Neural Syst Rehabil Eng 27(2):118–128
Rosin B et al (2011) closed-loop deep brain stimulation is superior in ameliorating parkinsonism. Neuron. 72(2):370–384
Hell F et al (2019) Deep brain stimulation programming 2.0 future perspectives for target identification and adaptive closed loop stimulation. Front Neurol. 10(314):1–11
Mugge L, Krafcik B, Pontasch G, Alnemari A, Neimat J, Gaudin D (2019) A review of biomarkers use in Parkinson with deep brain stimulation: a successful past promising a bright future. World Neurosurg 123:197–207
Yoshida F, Martinez-Torres I, Pogosyan A, Holl E, Petersen E, Chen CC, Foltynie T, Limousin P, Zrinzo LU, Hariz MI, Brown P (2010) Value of subthalamic nucleus local field potentials recordings in predicting stimulation parameters for deep brain stimulation in Parkinson’s disease. J Neurol Neurosurg Psychiatry 81(8):885–9
Nakao N, Nakai E, Nakai K, Itakura T (1999) Ablation of the subthalamic nucleus supports the survival of nigral dopaminergic neurons after nigrostriatal lesions induced by the mitochondrial toxin 3-nitropropionic acid. Ann Neurol 45(5):640–51
Spieles-Engemann AL, Behbehani MM, Collier TJ, Wohlgenant SL, Steece-Collier K, Paumier K, Daley BF, Gombash S, Madhavan L, Mandybur GT, Lipton JW (2010) Stimulation of the rat subthalamic nucleus is neuroprotective following significant nigral dopamine neuron loss. Neurobiol Dis 39(1):105–15
Temel Y, Visser-Vandewalle V, Kaplan S, Kozan R, Daemen MA, Blokland A, Schmitz C, Steinbusch HW (2006) Protection of nigral cell death by bilateral subthalamic nucleus stimulation. Brain Res 1120(1):100–5
Wallace BA, Ashkan K, Heise CE, Foote KD, Torres N, Mitrofanis J, Benabid AL (2007) Survival of midbrain dopaminergic cells after lesion or deep brain stimulation of the subthalamic nucleus in MPTP-treated monkeys. Brain 130(8):2129–45
Maesawa S, Kaneoke Y, Kajita Y, Usui N, Misawa N, Nakayama A, Yoshida J (2004) Long-term stimulation of the subthalamic nucleus in hemiparkinsonian rats: neuroprotection of dopaminergic neurons. J Neurosurg 100(4):679–87
Jakobs M, Fomenko A, Lozano AM, Kiening KL (2019) Cellular, molecular, and clinical mechanisms of action of deep brain stimulation—a systematic review on established indications and outlook on future developments. EMBO Mol Med 11(4)
Piallat B, Benazzouz A, Benabid AL (1996) Subthalamic nucleus lesion in rats prevents dopaminergic nigral neuron degeneration after striatal 6-OHDA injection: behavioural and immunohistochemical studies. Eur J Neurosci 8(7):1408–14
Meredith GE, Kang UJ (2006) Behavioral models of Parkinson’s disease in rodents: a new look at an old problem. Mov Disord 21(10):1595–606
Spieles-Engemann AL, Collier TJ, Sortwell CE (2010) A functionally relevant and long-term model of deep brain stimulation of the rat subthalamic nucleus: advantages and considerations. Eur J Neurosci 32(7):1092–9
Koprich JB, Johnston TH, Reyes MG, Sun X, Brotchie JM (2010) Expression of human A53T alpha-synuclein in the rat substantia nigra using a novel AAV1/2 vector produces a rapidly evolving pathology with protein aggregation, dystrophic neurite architecture and nigrostriatal degeneration with potential to model the pathology of Parkinson’s disease. Mol Neurodegener. 5(1):43
Koprich JB et al (2011) Progressive neurodegeneration or endogenous compensation in an animal model of Parkinson’s disease produced by decreasing doses of alphasynuclein. PLoS One 6(3):e17698
Musacchio T, Rebenstorff M, Fluri F, Brotchie JM, Volkmann J, Koprich JB, Ip CW (2017) Subthalamic nucleus deep brain stimulation is neuroprotective in the A53T α-synuclein Parkinson’s disease rat model. Ann Neurol 81(6):825–36
Blandini F, Armentero MT, Martignoni E (2008) The 6-hydroxydopamine model: news from the past. Parkinsonism Relat Disord 14:S124-9
Bové J, Prou D, Perier C, Przedborski S (2005) Toxin-induced models of Parkinson’s disease. NeuroRx 2(3):484–94
Amoozegar S, Pooyan M, Roughani M (2019) Toward a closed-loop deep brain stimulation in Parkinson’s disease using local field potential in parkinsonian rat model. Med Hypotheses 132:109360
Joghataei MT, Roghani M, Negahdar F, Hashemi L (2004) Protective effect of caffeine against neurodegeneration in a model of Parkinson’s disease in rat: behavioral and histochemical evidence. Parkinsonism Relat Disord 10(8):465–8
Baluchnejadmojarad T, Roghani M (2004) Evaluation of functional asymmetry in rats with dose-dependent lesions of dopaminergic nigrostriatal system using elevated body swing test. Physiol Behav 82(2–3):369–373
Roghani M, Niknam A, Jalali-Nadoushan MR, Kiasalari Z, Khalili M, Baluchnejadmojarad T (2010) Oral pelargonidin exerts dose-dependent neuroprotection in 6-hydroxydopamine rat model of hemi-parkinsonism. Brain Res Bull 82(5–6):279 (283)
Hilborn Robert C (2000) Chaos and nonlinear dynamics: an introduction for scientists and engineers. Oxford University Press on Demand
Iasemidis LD, Sackellares JC, Zaveri HP, Williams WJ (1990) Phase space topography and the Lyapunov exponent of electrocorticograms in partial seizures. Brain Topogr 2(3):187–201
Borg FG (2005) Review of nonlinear methods and modelling. arXiv preprint physics/0503026
Corron NJ, Hayes ST, Pethel SD, Blakely JN (2006) Chaos without nonlinear dynamics. Phys Rev Lett 97(2):024101
Wolf A, Swift JB, Swinney HL, Vastano JA (1985) Determining Lyapunov exponents from a time series. Physica D Nonlinear Phenomena. 16(3):285–317
TenBroek TM, Van Emmerik RE, Hasson CJ, Hamill J (2007) Lyapunov exponent estimation for human gait acceleration signals. J Biomech 40(2):S210
Rosenstein MT, Collins JJ, De Luca CJ (1993) A practical method for calculating largest Lyapunov exponents from small data sets. Physica D Nonlinear Phenomena 65(1–2):117–34
Chang JS, Kim EY, Jung D, Jeong SH, Kim Y, Roh MS, Ahn YM, Hahm BJ (2015) Altered cardiorespiratory coupling in young male adults with excessive online gaming. Biol Psychol 110:159–66
Fukunaga K (1972) Introduction to statistical pattern recognition, 2nd edn. Academic Press, New York
E. Ebrahimzadeh, M. S. Manuchehri, S. Amoozegar, B. N. Araabi, H (2017) Soltanian-Zadeh “A time local subset feature selection for prediction of sudden cardiac death from ECG signal”. Med Biol Eng Comput 56(7) https://doi.org/10.1007/s11517-017-1764-1
Ebrahimzadeh E, Shams M, Rahimpour A, Fayaz F, Mirbagheri M, Hakimi N, HashemiFesharaki SS, Soltanian-Zadeh H (2019) Epilepsy presurgical evaluation of patients with complex source localization by a novel component-based EEG-fMRI approach. Iran J Radiol 16(1):e99134. https://doi.org/10.5812/iranjradiol.99134
Ebrahimzadeh ME, Shams A (2020) Rahimpour Jounghani, et al. Localizing confined epileptic foci in patients with an unclear focus or presumed multifocality using a component-based EEG-fMRI method. Cogn Neurodyn https://doi.org/10.1007/s11571-020-09614-5
Amoozegar S, Pooyan M, Ebrahimzadeh E (2013) Classification of brain signals in normal subjects and patients with epilepsy using mixture of experts. Int J Eng Intell Syst Electr Eng Commun 4(1):1–8
Ebrahimzadeh E, Amoozegar S, Asgarinejad MM, Dolatabad MR, Bagheri M, Soroush MZ (2019) Simultaneous EEG-fMRI: a multimodality approach to localize the seizure onset zone in patients with epilepsy. Int J Biol Med 1:130–139. https://doi.org/10.36811/ijbm.2019.110017
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Amoozegar, S., Pooyan, M. & Roghani, M. Identification of effective features of LFP signal for making closed-loop deep brain stimulation in parkinsonian rats. Med Biol Eng Comput 60, 135–149 (2022). https://doi.org/10.1007/s11517-021-02470-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11517-021-02470-3