Abstract
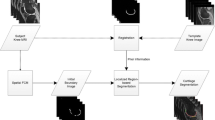
Quantitative thickness computation of knee cartilage in ultrasound images requires segmentation of a monotonous hypoechoic band between the soft tissue-cartilage interface and the cartilage-bone interface. Speckle noise and intensity bias captured in the ultrasound images often complicates the segmentation task. This paper presents knee cartilage segmentation using locally statistical level set method (LSLSM) and thickness computation using normal distance. Comparison on several level set methods in the attempt of segmenting the knee cartilage shows that LSLSM yields a more satisfactory result. When LSLSM was applied to 80 datasets, the qualitative segmentation assessment indicates a substantial agreement with Cohen’s κ coefficient of 0.73. The quantitative validation metrics of Dice similarity coefficient and Hausdorff distance have average values of 0.91 ± 0.01 and 6.21 ± 0.59 pixels, respectively. These satisfactory segmentation results are making the true thickness between two interfaces of the cartilage possible to be computed based on the segmented images. The measured cartilage thickness ranged from 1.35 to 2.42 mm with an average value of 1.97 ± 0.11 mm, reflecting the robustness of the segmentation algorithm to various cartilage thickness. These results indicate a potential application of the methods described for assessment of cartilage degeneration where changes in the cartilage thickness can be quantified over time by comparing the true thickness at a certain time interval.







Similar content being viewed by others
References
Abraham AM, Goff I, Pearce MS, Francis RM, Birrell F (2011) Reliability and validity of ultrasound imaging of features of knee osteoarthritis in the community. BMC Muscuskelet Disord 70(12):1471–2474
Aisen AM, McCune WJ, MacGuire A, Carson PL, Silver TM, Jafri SZ, Martel W (1984) Sonographic evaluation of the cartilage of the knee. Radiology 153(3):781–784
Buckland-Wright JC (1994) Quantitative radiography of osteoarthritis. Ann Rheum Dis 53:268–275
Chan TF, Vese LA (2001) Active contours without edges. IEEE Trans Image Process 10(2):266–277
Cohen J (1960) A coefficient of agreement for nominal scales. Educ Psychol Meas 20(1):37–46
Dodin P, Pelletier JP, Martel-Pelletier J, Abram F (2010) Automatic human knee cartilage segmentation from 3-D magnetic resonance images. IEEE Trans Biomed Eng 57(11):2699–2711
Folkesson J, Dam EB, Olsen OF, Pettersen PC, Christiansen C (2007) Segmenting articular cartilage automatically using a voxel classification approach. IEEE Trans Biomed Eng 26(1):106–115
Fripp J, Crozier S, Warfield SK, Ourselin S (2010) Automatic segmentation and quantitative analysis of the articular cartilages from magnetic resonance images of the knee. IEEE Trans Med Imag 29(1):55–63
Graichen H, Eisenhart-Rothe R, Vogl T, Englmeier KH, Eckstein F (2004) Quantitative assessment of cartilage status in osteoarthritis by quantitative magnetic resonance imaging. Arthritis Rheum 50:811–816
Heuer F, Sommer M, III JBR, Bottlang M (2001) Estimation of cartilage thickness from joint surface scans: comparative analysis of computational methods. In: Proceedings 2001 ASME bioengineering Conference, vol 50, pp 569–570
Iagnocco A, Coari G, Zoppini A (1992) Sonographic evaluation of femoral condylar cartilage in osteoarthritis and rheumatoid arthritis. Scand J Rheumatol 21(4):201–203
Jackson D, Simon T, Aberman H (2001) Symptomatic articular cartilage degeneration: the impact in the new millennium. Clin Orthop Relat Res 391:14–15
Kazam JK, Nazarian LN, Miller TT, Sofka CM, Parker L, Adler RS (2011) Sonographic evaluation of femoral trochlear cartilage in patients with knee pain. J Ultrasound Med 30(6):797–802
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33 (1):159–174
Li C, Huang R, Ding Z, Gatenby C, Metaxas DN, Gore JC (2011) A level set method for image segmentation in the presence of intensity inhomogeneities with application to MRI. IEEE Trans Image Process 20 (7):2007–2016
Maurer CR, Qi R, Raghavan V (2003) A linear time algorithm for computing exact Euclidean distance transforms of binary images in arbitrary dimensions. IEEE Trans Pattern Anal Mach Intell 25(2):265–270
Moller I, Bong D, Naredo E, Filippucci E, Carrasco I, Moragues C, Iagnocco A (2008) Ultrasound in the study and monitoring of osteoarthritis. Osteoarthr Cartil 16:S4–S7
Mukherjee S, Acton ST (2015) Region based segmentation in presence of intensity inhomogeneity using legendre polynomials. IEEE Signal Process Lett 22(3):298–302
Naredo E, Acebes C, Moller I, Canillas F, de Agustin JJ, de Miguel E, Filippucci E, Iagnocco A, Moragues C, Tuneu R, Uson J, Garrido J, Delgado-Baeza E, Saenz-Navarro I (2009) Ultrasound validity in the measurement of knee cartilage thickness. Ann Rheum Dis 68:1322–1327
Pakin SK, Tamez-Pena JG, Totterman S, Parker KJ (2002) Segmentation, surface extraction, and thickness computation of articular cartilage. In: Proceedings SPIE Medical Imaging 2002: Image Processing, San Diego, USA, vol 4686, pp 155–166
Roemer FW, Crema MD, Trattnig S, Guermazi A (2011) Advances in imaging of osteoarthritis and cartilage. Radiology 260(2):332–354
Solloway S, Hutchison C, Waterton J, Taylor C (1993) The use of active shape models for making thickness measurements of articular cartilage from MR images. Magn Reson Med 13(5):943–952
Tang J, Millington S, Acton ST, Crandall J, Hurwitz S (2006) Surface extraction and thickness measurement of the articular cartilage from MR images using directional gradient vector flow snakes. IEEE Trans Biomed Eng 53(5):896–907
Wang J, Cheng Y, Guo C, Wang Y, Tamura S (2016) Shape-intensity prior level set combining probabilistic atlas and probability map constrains for automatic liver segmentation from abdominal CT images. Int J Comput Assist Radiol Surg 11(5):817–826
Wang L, He L, Mishra A, Li C (2009) Active contours driven by local Gaussian distribution fitting energy. Signal Process 89(12):2435–2447
Wang XF, Min H, Zou L, Zhang YG (2015) A novel level set method for image segmentation by incorporating local statistical analysis and global similarity measurement. Pattern Recogn 48(1):189–204
Xiao G, Brady M, Noble JA, Zhang Y (2002) Segmentation of ultrasound B-mode images with intensity inhomogeneity correction. IEEE Trans Med Imag 21(1):48–57
Yezzi A, Prince J (2003) An eulerian PDE approach for computing tissue thickness. IEEE Trans Med Imag 22(10):1332–1339
Yin Y, Zhang X, Williams R, Wu X, Anderson DD, Sonka M (2010) LOGISMOS–Layered optimal graph image segmentation of multiple objects and surfaces: cartilage segmentation in the knee joint. IEEE Trans Med Imag 29(12):2023–2037
Zhang K, Zhang L, Zhang S (2010) A variational multiphase level set approach to simultaneous segmentation and bias correction. In: Proceedings IEEE International Conference Image Processing, Hong Kong, pp 4105–4108
Zhang K, Zhang L, Song H, Zhang D (2013) Reinitialization-free level set evolution via reaction diffusion. IEEE Trans Image Process 22(1):258–271
Zhang K, Zhang L, Lam KM, Zhang D (2016) A level set approach to image segmentation with intensity inhomogeneity. IEEE Trans Cybern 46(2):546–557
Acknowledgements
This work was supported in part by the University of Malaya Research Grant (RP020A-13AET), in part by the International Graduate Research Assistantship Scheme, and in part by the Postgraduate Research Grant (PG003-2014B).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Faisal, A., Ng, SC., Goh, SL. et al. Knee cartilage segmentation and thickness computation from ultrasound images. Med Biol Eng Comput 56, 657–669 (2018). https://doi.org/10.1007/s11517-017-1710-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11517-017-1710-2