Abstract
Background
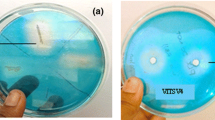
Screening of isolates for their potency to produce streptokinase was an important criterion of this research. The current study emphasizes the strain improvement, optimization and purification studies for enhanced production of streptokinase from Streptococcus uberis TNA-M1 isolated from bovine milk.
Methods
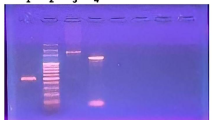
The study was carried out on samples collected from milk sample. Primary screening and characterization is used as an excellent source for the isolation of β-hemolytic organisms. Strain improvement was done by both physical & chemical mutagenesis. The enzyme activity was checked by clot lysis assay and confirmed by fibrin plate method. The partially purified and crude enzyme were analysed by high-performance liquid chromatography. Molecular weight & enzyme purity was checked by SDS–PAGE, further confirmed by fibrin zymography.
Results
Out of the 3 isolated strains, only one isolate expressed β-haemolysis with streptokinase (SK) activity. Based on the results of radial caseinolytic assay and blood clot dissolving assay, isolate TNA-M1 demonstrated the highest streptokinase activity. Based on morphological, biochemical and molecular characterization, it was identified as Streptococcus uberis and the strain was named as Streptococcus uberis TNA-M1. The results indicated that ultra-violet (UV) and ethyl methane sulfonate (EMS) were effective mutagenic agents for strain improvement of Streptococcus uberis TNA-M1 and enhanced SK productivity. HPLC analysis was performed in order to confirm the presence of streptokinase with the similar retention time (0.875 min) with its standard (0.854) min. SDS-PAGE of the enzyme showed protein band of approximately 47 kDa and confirmed by fibrin zymography. It exhibited fibrinolytic activity, which was more potent than other fibrinolytic enzymes. Glucose and peptone were recorded to be the optimum carbon and nitrogen sources respectively.
Conclusion
Thus this study presents its novelty by highlighting the potential of Streptococcus uberis TNA-M1 as a significant source for the production of fibrinolytic enzymes.
Similar content being viewed by others
References
Aradhye P K, Chavan M D (2015). Production, charecterization and in-vitroStudy of fibrinolytic enzyme from locally isolated Micrococcus luteusB-07. World J Pharm Pharmaceut Sci, 4(8):775–783
Baewald G, Mayer G, Heikel R, Volzke K D, Roehlig RDecker, Koehler W, Gerllach D(1975) Fermentative Production of streptococcus metabolites, especially streptokinase. German patent DD, 111096
Banerjee A, Chisti Y, Banerjee U C (2004). Streptokinase–a clinically useful thrombolytic agent. Biotechnol Adv, 22(4): 287–307
Blix S (1962). the effectiveness of the activators in clot lysis with special reference to fibrinolytic therapy: a new method for the determination of performed clot lysis. Acta Med Scand, 172: 386
Boersma E, Mercado N, Poldermans D, Gardien M, Vos J, Simoons M L (2003). Acute myocardial infarction. Lancet, 361(9360): 847–858
Boland A, Dundar Y, Bagust A, Haycox A, Hill R, Mujica Mota R, Walley T, Dickson R (2003). Early thrombolysis for the treatment of acute myocardial infarction: a systematic review and economic evaluation. Health Technol Assess, 7(15): 1–136
Christensen L R, Macleod C M (1945). A proteolytic enzyme of se-rum: characterization, activation, and reaction with inhibitors. J Gen Physiol, 28(6): 559–583
Chung D M, Kim K E, Ahn K H, Park C S, Kim D H, Koh H B, Chun H K, Yoon B D, Kim H J, Kim M S, Choi N S (2011). Silver-stained fibrin zymography: separation of proteases and activity detection using a single substrate-containing gel. Biotechnol Lett, 33(8): 1663–1666
Feldman L J (1974) Streptokinase manufacture. In German.German patent DE 2354019.
Grimont P A D, Grimont F (1984). Bergey’s Manual of systematic bacteriology. Baltimore: Williams and Wilkins.1.477–484
Holmström B, Seppälä P, Vilhunen R, Enari T M (1965). Streptokinase assay on large agar diffusion plates. Acta Chem Scand, 19(7): 1549–1554
Huangkai Z, Shenghan G, Wei-Hua C (2012). EvolView, An online tool for visualizing, annotating and managing phylogenetic trees. Nucleic Acids Res, 40(W1): 569–572
Hyun H H, Lee Y B, Song K H, Jeon J Y, Lee H H (1997). Strain improvement for enhanced production of streptokinase and streptodornase in Streptococcus sp. J Microbiol Biotechnol, 7: 101–106
Ibrahim S A, O’Sullivan D J (2000). Use of chemical mutagenesis for the isolation of food grade ß-galactosidase overproducing mutants of bifidobacteria, lactobacilli and Streptococcus thermophilus. J Dairy Sci, 83(5): 923–930
Kumar A, Pulicherla K, Ram K S, Rao K R S (2011). Evolutionary trend of thrombolytic?their significance, Int J Biosci Biotech, 3(1): 1–18
Laemmli V K (1970). Determination of protein molecular weight in polyacrylamide gels. Nature, 227: 680–685
Lowry O H, Rosebrough N J, Farr A L, Randall R J (1951). Protein measurement with the Folin phenol reagent. J Biol Chem, 193(1): 265–275
Madhuri D H, Madhuri M, Neha A S, Mohanasrinivasan V, Subathra D C (2011) studies on screening, strain development, production and optimization of streptokinase from beta hemolytic streptococci. World J SciTechnol, 1(3):7–11
Magarvey N A, Keller J M, Bernan V, Dworkin M, Sherman D H (2004). Isolation and characterization of novel marine-derived actinomycete taxa rich in bioactive metabolites. Appl Environ Microbiol, 70(12): 7520–7529
Parekh S Vinci VA, Strobel R J, (2004). Improvement of microbial strains and fermentation processes. App Microbiol Biotechnol, 54287–301
Rodríguez P, Fuentes P, Barro M, Alvarez J G, Muñoz E, Collen D, Lijnen H R (1995). Structural domains of streptokinase involved in the interaction with plasminogen. Eur J Biochem, 229(1): 83–90
Schick L A, Castellino F J (1974). Direct evidence for the generation of an active site in the plasminogen moiety of the streptokinase-human plasminogen activator complex. Biochem Biophys Res Commun, 57 (1): 47–54
Taleb T, Kunameeni A, Ellaiah P (2005). Isolation and mutagenesis of streptokinase producing bacteria. Am J Immunol, 1(4): 125–129
Tillett W S, Garner R L (1933). Thefibrinolytic activity of hemolytic streptococci. J Exp Med, 58(4): 485–502
Tough J (2005). Thrombolytic therapy in acute myocardial infarction. Nurs Stand, 19(37): 55–64, quiz 66
Uversky V N, Fink A L (2004). Conformational constraints for amyloid fibrillation: the importance of being unfolded. Biochim Biophys Acta, 1698(2): 131–153
WHO (2010) Burden: mortality, morbidity and risk factors. In: Global status report on non-communicable diseases, WHO Press, Geneva. pp, 9–10
Acknowledgements
We are greatly indebted to Vellore Institute of Technology for the constant encouragement, help and support for extending necessary facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghosal, T., Augustine, N., Siddapur, A. et al. Strain improvement, optimization and purification studies for enhanced production of streptokinase from Streptococcus uberis TNA-M1. Front. Biol. 12, 376–384 (2017). https://doi.org/10.1007/s11515-017-1467-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11515-017-1467-x