Abstract
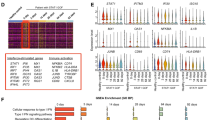
Analysis of gut barrier status, monocyte and lymphocyte activation and T regulatory (Treg) cells at diagnosis before and after therapy, in patients with multiple sclerosis (MS). Analysis of differential effects of interferon beta (IFN-β), glatiramer acetate (GA) and natalizumab. Thirty-five patients with untreated MS were included. Gut barrier status (serum concentrations of intestinal fatty acid binding protein), monocyte (serum levels of soluble CD14, soluble CD163 and interleukin 6) and T lymphocyte activation (CD4 + DR+ and CD8 + DR+) and Treg (CD4 + CD25highFoxP3+) cells were analyzed. Patients with clinical isolated syndrome and relapsing-remitting forms were treated with IFN-β or GA, and immune characteristics were reevaluated following up after 6 months. A sample of 56 stable RR MS patients, in treatment with IFN-β, GA or natalizumab, and 50 healthy individuals were included as controls. Gut barrier status was similar in MS patients and healthy controls. Untreated patients with relapsing-remitting and primary progressive patterns of MS showed increased serum levels of soluble CD14. At baseline, significant increases in activated T lymphocytes and Treg were detected in patients. A significant decrease of CD4 + DR+, CD8 + DR+, and Treg percentages after 6 months of therapy was observed. In previously treated patients, IFN-β, GA, or natalizumab therapies were associated with a comparable cell proportion of activated lymphocytes and Treg. MS patients have a baseline state characterized by monocyte and lymphocyte activation, not related with gut barrier lesion. An increase in Treg number, correlated with activated T CD8+ lymphocytes, was detected. Treatment with IFN-β, GA or natalizumab was associated with a comparable decrease in activated lymphocytes and Treg.

ᅟ



Similar content being viewed by others
References
Bailey MT, Dowd SE, Galley JD, Hufnagle AR, Allen RG, Lyte M (2011) Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor-induced immunomodulation. Brain Behav Immun 25:397–407
Bas S, Gauthier BR, Spenato U, Stingelin S, Gabay C (2004) CD14 is an acute phase protein. J Immunol 172:4470–4479
Brettschneider J, Ecker D, Bitsch A, Bahner D, Bogumil T, Dressel A, Elitok E, Kitze B, Poser S, Weber F, Tumani H (2002) The macrophage activity marker sCD14 is increased in patients with multiple sclerosis and upregulated by interferon beta-1b. J Neuroimmunol 133:193–197
Chen YC, Yang X, Miao L, Liu ZG, Li W, Zhao ZX, Sun XJ, Jiang GX, Chen SD, Cheng Q (2012) Serum level of interleukin-6 in Chinese patients with multiple sclerosis. J Neuroimmunol 249:109–111
Chi LJ, Wang HB, Zhang Y, Wang WZ (2007) Abnormality of circulating CD4(+)CD25(+) regulatory T cell in patients with Guillain-Barre syndrome. J Neuroimmunol 192:206–214
Crack PJ, Bray PJ (2007) Toll-like receptors in the brain and their potential roles in neuropathology. Immunol Cell Biol 85:476–480
Dalla Libera D, Di Mitri D, Bergami A, Centonze D, Gasperini C, Grasso MG, Galgani S, Martinelli V, Comi G, Avolio C, Martino G, Borsellino G, Sallusto F, Battistini L, Furlan R (2011) T regulatory cells are markers of disease activity in multiple sclerosis patients. PLoS One 6:e21386
Fabriek BO, Møller HJ, Vloet RP, van Winsen LM, Hanemaaijer R, Teunissen CE, Uitdehaag BM, van den Berg TK, Dijkstra CD (2007) Proteolytic shedding of the macrophage scavenger receptor CD163 in multiple sclerosis. J Neuroimmunol 187:179–186
Fabriek BO, van Bruggen R, Deng DM, Ligtenberg AJ, Nazmi K, Schornagel K, Vloet RP, Dijkstra CD, van den Berg TK (2009) The macrophage scavenger receptor CD163 functions as an innate immune sensor for bacteria. Blood 113:887–892
Fleck AK, Schuppan D, Wiendl H, Klotz L (2017) Gut-CNS-Axis as possibility to modulate inflammatory disease activity-implications for multiple sclerosis. Int J Mol Sci 18:E1526
Haas J, Hug A, Viehöver A, Fritzsching B, Falk CS, Filser A, Vetter T, Milkova L, Korporal M, Fritz B, Storch-Hagenlocher B, Krammer PH, Suri-Payer E, Wildemann B (2005) Reduced suppressive effect of CD4+CD25high regulatory T cells on the T cell immune response against myelin oligodendrocyte glycoprotein in patients with multiple sclerosis. Eur J Immunol 35:3343–3352
Hellings N, Barée M, Verhoeven C, D'hooghe MB, Medaer R, Bernard C, Raus J, Stinissen P (2001) T-cell reactivity to multiple myelin antigens in multiple sclerosis patients and healthy controls. J Neurosci Res 63:290–302
Hemmer B, Kerschensteiner M, Korn T (2015) Role of the innate and adaptive immune responses in the course of multiple sclerosis. Lancet Neurol 14:406–419
Henderson AP, Barnett MH, Parratt JD, Prineas JW (2009) Multiple sclerosis: distribution of inflammatory cells in newly forming lesions. Ann Neurol 66:739–753
Huan J, Culbertson N, Spencer L, Bartholomew R, Burrows GG, Chou YK, Bourdette D, Ziegler SF, Offner H, Vandenbark AA (2005) Decreased FOXP3 levels in multiple sclerosis patients. J Neurosci Res 81:45–52
Jones AP, Kermode AG, Lucas RM, Carroll WM, Nolan D, Hart PH (2017) Circulating immune cells in multiple sclerosis. Clin Exp Immunol 187:193–203
Josefowicz SZ, Lu LF, Rudensky AY (2012) Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol 30:531–564
Karp CL, Biron CA, Irani DN (2000) Interferon beta in multiple sclerosis: is IL-12 suppression the key? Immunol Today 21:24–28
Lalive PH, Neuhaus O, Benkhoucha M, Burger D, Hohlfeld R, Zamvil SS, Weber MS (2011) Glatiramer acetate in the treatment of multiple sclerosis: emerging concepts regarding its mechanism of action. CNS Drugs 25:401–414
Litvack ML, Palaniyar N (2010) Soluble innate immune pattern-recognition proteins for clearing dying cells and cellular components: implications on exacerbating or resolving inflammation. Innate Immun 16:191–200
Lutterotti A, Kuenz B, Gredler V, Khalil M, Ehling R, Gneiss C, Egg R, Deisenhammer F, Berger T, Reindl M (2006) Increased serum levels of soluble CD14 indicate stable multiple sclerosis. J Neuroimmunol 181:145–149
Matsushita T, Tateishi T, Isobe N, Yonekawa T, Yamasaki R, Matsuse D, Murai H, Kira J (2013) Characteristic cerebrospinal fluid cytokine/chemokine profiles in Neuromyelitis Optica, relapsing remitting or primary progressive multiple sclerosis. PLoS One 8:e61835
Michel L, Berthelot L, Pettré S, Wiertlewski S, Lefrère F, Braudeau C, Brouard S, Soulillou JP, Laplaud DA (2008) Patients with relapsing-remitting multiple sclerosis have normal Treg function when cells expressing IL-7 receptor alpha-chain are excluded from the analysis. J Clin Invest 118:3411–3419
Noori-Zadeh A, Mesbah-Namin SA, Bistoon-Beigloo S, Bakhtiyari S, Abbaszadeh HA, Darabi S, Rajabibazl M, Abdanipour A (2016) Regulatory T cell number in multiple sclerosis patients: a meta-analysis. Mult Scler Relat Disord 5:73–76
Piton G, Capellier G (2016) Biomarkers of gut barrier failure in the ICU. Curr Opin Crit Care 22:152–160
Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, Fujihara K, Havrdova E, Hutchinson M, Kappos L, Lublin FD, Montalban X, O'Connor P, Sandberg-Wollheim M, Thompson AJ, Waubant E, Weinshenker B, Wolinsky JS (2011) Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 69:292–302
Prodinger C, Bunse J, Krüger M, Schiefenhövel F, Brandt C, Laman JD, Greter M, Immig K, Heppner F, Becher B, Bechmann I (2011) CD11c-expressing cells reside in the juxtavascular parenchyma and extend processes into the glia limitans of the mouse nervous system. Acta Neuropathol 121:445–458
Putzki N, Baranwal MK, Tettenborn B, Limmroth V, Kreuzfelder E (2010) Effects of natalizumab on circulating B cells, T regulatory cells and natural killer cells. Eur Neurol 63:311–317
Ríos-Toro JJ, Márquez-Coello M, García-Álvarez JM, Martín-Aspas A, Rivera-Fernández R, Sáez de Benito A, Girón-González JA (2017) Soluble membrane receptors, interleukin 6, procalcitonin and C reactive protein as prognostic markers in patients with severe sepsis and septic shock. PLoS One 12:e0175254
Romme Christensen J, Bornsen L, Ratzer R, Piehl F, Khademi M, Olsson T, Sørensen PS, Sellebjerg F (2013) Systemic inflammation in progressive multiple sclerosis involves follicular T-helper, Th17- and activated B-cells and correlates with progression. PLoS One 8:e57820
Stilund M, Reuschlein AK, Christensen T, Møller HJ, Rasmussen PV, Petersen T (2014) Soluble CD163 as a marker of macrophage activity in newly diagnosed patients with multiple sclerosis. PLoS One 9:e98588
Stüve O, Gold R, Chan A, Mix E, Zettl U, Kieseier BC (2008) alpha4-integrin antagonism with natalizumab: effects and adverse effects. J Neurol 255 Suppl 6:58–65
van Mierlo GJ, Scherer HU, Hameetman M, Morgan ME, Flierman R, Huizinga TW, Toes RE (2008) Cutting edge: TNFR-shedding by CD4 + CD25+ regulatory T cells inhibits the induction of inflammatory mediators. J Immunol 180:2747–2751
Venken K, Hellings N, Hensen K, Rummens JL, Medaer R, D'hooghe MB, Dubois B, Raus J, Stinissen P (2006) Secondary progressive in contrast to relapsing-remitting multiple sclerosis patients show a normal CD4+CD25+ regulatory T-cell function and FOXP3 expression. J Neurosci Res 83:1432–1446
Yadav SK, Mindur JE, Ito K, Dhib-Jalbut S (2015) Advances in the immunopathogenesis of multiple sclerosis. Curr Opin Neurol 28:206–219
Contributions Made by each of the Authors to the Article
Conceived and designed the experiments: María Carmen González-Oria, Mercedes Márquez-Coello, José A Girón-González.
Clinical follow-up of patients`: María Carmen González-Oria, Joaquín Argente-Alcaraz, Miguel Moya-Molina.
Contributed reagents/materials/analysis tools: Mercedes Márquez-Coello; José Antonio Girón-González.
Analyzed the data: María Carmen González-Oria, José Antonio Girón-Ortega, José Antonio Girón-González.
Wrote the draft: José Antonio Girón-Ortega, José Antonio Girón-González.
All authors contributed to conception of the study, and critical revision of the manuscript, and saw and approved the final version.
Availability of Data and Materials
All data generated or analyzed during this study are included in this published article.
Funding
This work was supported by a Grant from Neurología, Fundación para la Investigación Biomédica de Cádiz. MCGO was the recipient of this Grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
The project was approved by the hospital ethical research committee. Written informed consent was obtained from each participant.
Consent for Publication
Not applicable.
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
González-Oria, M.C., Márquez-Coello, M., Girón-Ortega, J.A. et al. Monocyte and Lymphocyte Activation and Regulation in Multiple Sclerosis Patients. Therapy Effects. J Neuroimmune Pharmacol 14, 413–422 (2019). https://doi.org/10.1007/s11481-018-09832-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11481-018-09832-z