Abstract
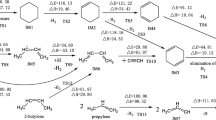
The mechanism of the multiple-pathway and multiple-step degradation reactions of 2,3,7,8-tetrachlorinated dibenzo-p-dioxins with H and Cl atoms is investigated using the density functional theory. The electronic structures and the minimum energy path (MEP) are calculated at the B3LYP/6-311+G(d,p) level, and energetic information (single-point) is further refined at the B3LYP/6-311++G(3df,2p) level. The main possible ring opening reaction pathways of the C-O bond breakdown include indirect cleavage ring opening reaction, hydrogen addition reaction, hydrogen addition elimination reaction, and chlorine addition elimination reaction. Ten reaction steps of the six reaction pathways are considered. Our calculations indicate that hydrogen addition reaction step TS1c is of the smallest barrier height, and indirect cleavage ring opening reaction channel has the largest barrier height and is the most endothermic.
Similar content being viewed by others
References
Duan H B, Li J H, Liu Y C, et al. Characterization and inventory of pcdd/fs and pbdd/fs emissions from the incineration of waste printed circuit board. Environ Sci Technol, 2011, 45: 6322–6328
Tang Z W, Huang Q F, Yang Y F. PCDD/Fs in fly ash from waste incineration in china: a need for effective risk management. Environ Sci Technol, 2013, 47: 5520–5521
Vejerano E P, Holder A L, Marr L C. Emissions of polycyclic aromatic hydrocarbons, polychlorinated dibenzo-p-dioxins, and dibenzofurans from incineration of nano-materials. Environ Sci Technol, 2013, 47: 4866–4874
Lin C J, Hsu J F, Liao P C. Co-exposure of dioxin-like polychlorin ated biphenyls and polychlorinated dibenzo-p-dioxins and dibenzofurans in free-range hens and implications derived from congener profile analysis. J Agr Food Chem, 2012, 60: 1963–1972
Hites R A. Dioxins: An overview and history. Environ Sci Technol, 2011, 45: 16–20
Burke K. Perspective on density functional theory. J Chem Phys, 2012, 36: 150901
Shi J C, Zuo R, Meng S C. DFT study on adduct reaction paths of GaN MOCVD growth. Sci China Tech Sci, 2013, 56: 1644–1650
Xia X Q, Zhang H, Zhang G L. Theoretical studies of structures and spectroscopic properties of [(tpy)(bpy)RuC ≡ CC6H4R]+(tpy=2,2′:6′, 2″-terpyridine, bpy=2,2′-bipyridine; R=F, Cl, H, Me and OMe). Sci Sci China Tech Sci, 2014, 57: 725–733
Chrétien S, Metiu H. DFT study of the electronic properties of laocl surfaces. J Phys Chem C, 2012, 116: 681–691
Long H, Pivovar B. Hydroxide degradation pathways for imidazolium cations: a dft study. J Phys Chem C, 2014, 118: 9880–9888
Yu W N, Hu J T, Xu F, et al. Mechanism and direct kinetics study on the homogeneous gas-phase formation of PBDD/Fs from 2-BP, 2,4-DBP, and 2,4,6-TBP as Precursors. Environ Sci Technol, 2011, 45: 1917–1925
Liu G S, Yu J G. Theoretical study on the structure, vibrational frequency and thermodynamic properties of 2,3,7,8-tetrachlorinated dibenzo-p-dioxin. J Mol Struct, 2005, 717: 15–19
Xu F, Wang H, Zhang Q Z, et al. Kinetic properties for the complete series reactions of chlorophenols with oh radicals-relevance for dioxin formation. Environ Sci Technol, 2010, 44: 1399–1404
Zhang Q Z, Yu W N, Zhang R X, et al. Quantum chemical and kinetic study on dioxin formation from the 2,4,6-tcp and 2,4-dcp precursors. Environ Sci Technol, 2010, 44: 3395–3403
Yu W N, Hu J T, Xu F, et al. Mechanism and direct kinetics study on the homogeneous gas-phase formation of pbdd/fs from 2-bp, 2,4-dbp, and 2,4,6-tbp as precursors. Environ Sci Technol, 2011, 45: 1917–1925
Xu F, Yu W N, Zhou Q, et al. Mechanism and direct kinetic study of the polychlorinated dibenzo-p-dioxin and dibenzofuran formations from the radical/radical cross-condensation of 2,4-dichlorophenoxy with 2-chlorophenoxy and 2,4,6-trichlorophenoxy. Environ Sci Technol, 2011, 45: 643–650
Lee J E, Choi W, Mhin B J, et al. Theoretical study on the reaction of OH radicals with polychlorinated dibenzo-p-dioxin. J Phys Chem A, 2004, 108: 607–614
Zhang C X, Sun T L, Sun, X M. Mechanism for OH-initiated degradation of 2,3,7,8-tetrachlorinated dibenzo-p-dioxins in the presence of O2 and NO/H2O. Environ Sci Technol, 2011, 45: 4756–4762
Sun X M, Zhang C X, Zhao Y Y, et al. Atmospheric chemical reactions of 2,3,7,8-tetrachlorinated dibenzofuran initiated by an OH radical: Mechanism and kinetics. Environ Sci Technol, 2012, 46: 8148–8155
Kruse H, Goerigk L, Grimme S. Why the standard b3lyp/6-31g* model chemistry should not be used in dft calculations of molecular thermochemistry: understanding and correcting the problem. J Org Chem, 2012, 77: 10824–10834
Torres E, DiLabio G A. A (nearly) universally applicable method for modeling noncovalent interactions using b3lyp. J Phys Chem Lett, 2012, 3: 1738–1744
Jimenez-Izal E, Chiatti F, Corno M, et al. Glycine adsorption at nonstoichiometric (010) hydroxyapatite surfaces: a b3lyp study. J Phys Chem C, 2012, 116: 14561–14567
Lu L L, Hu H, Hou H, et al. An improved b3lyp method in the calculation of organic thermochemistry and reactivity. Comput Theor Chem, 2013, 1015: 64–71
Motoyuki S, Hiroshi F. A quantum generalization of intrinsic reaction coordinate using path integral centroid coordinates. J Chem Phys, 2012, 136: 184103
Frisch M J, Trucks G W, Schlegel H B, et al. Gaussian. Revision A.02. Wallingford (CT): Gaussian Inc., 2009
Liu Y Y, Wang W L, Zhang T L, et al. On the kinetic mechanism of the hydrogen and oxygen abstraction reactions of CH3S with HOO: A dual-level direct dynamics study. Comput Theor Chem, 2011, 964: 169–175
Wang L, Wen J M, He H Q, et al. A dual-level direct dynamics study on the hydrogen abstraction reaction of oxygen atom with methyl hydrazine. Int J Quantum Chem, 2013, 113: 2338–2344
Zhang H, Zhang G L, Liu J Y, et al. Dual-level direct dynamics studies on the reactions of tetramethylsilane with chlorine and bromine atoms. Theor Chem Acc, 2010, 125: 75–82
Hu W P, Truhlar D G. Factors affecting competitive ion-molecule reactions: ClO- + C2H5Cl and C2D5Cl via E2 and SN2 channels. J Am Chem Soc, 1996, 118: 860–869
Corchado J C, Chuang Y Y, Fast P L, et al. POLYRATE. version 9.7. Minneapolis (MN): Department of Chemistry and Supercomputer Institute, University of Minnesota. 2007
Truhlar D G, Garrett B C. Variational transition state theory. Accounts Chem Res, 1980, 13: 440–448
Truhlar D G, Isaacson A D, Garrett B C. Generalized Transition State Theory. In: Baer M, eds. The Theory of Chemical Reaction Dynamics. Boca Raton, FL: CRC Press, 1985. 65
Garrett B C, Truhlar D G. Criterion of minimum state density in the transition state theory of bimolecular reactions. J Chem Phys, 1979, 70: 1593–1598
Garrett B C, Truhlar D G. Generalized transition state theory. Bond Energy-Bond Order method for canonical variational calculations with applications to hydrogen atom transfer reactions. J Am Chem Soc, 1979, 101: 4534–4548
Garrett B C, Truhlar D G, Grev R S, et al. Improved treatment of threshold contributions in variational transition state theory. J Phys Chem, 1980, 84: 1730–1748
Lu D H, Truong T N, Melissas V S, et al. POLYRATE 4: A new version of a computer program for the calculation of chemical reaction rates for polyatomics. Comput Phys Commun, 1992, 71: 235–262
Liu Y P, Lynch G C, Truong T N, et al. Molecular modeling of the kinetic isotope effect for the [1,5]-sigma tropic rearrangement of cis-1,3-pentadiene. J Am Chem Soc, 1993, 115: 2408–2415
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Zhang, H., Zhang, K. & Sun, S. Theoretical study of the degradation mechanism on the reactions 2,3,7,8-tetrachlorinated dibenzo-p-dioxins with hydrogen and chlorine atoms. Sci. China Technol. Sci. 58, 181–188 (2015). https://doi.org/10.1007/s11431-014-5736-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11431-014-5736-5