Abstract
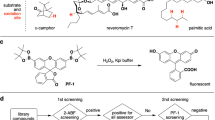
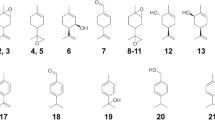
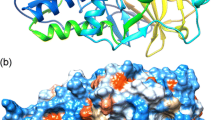
Directed evolution (DE) inspired by natural evolution (NE) has been achieving tremendous successes in protein/enzyme engineering. However, the conventional “one-protein-for-one-task” DE cannot match the “multi-proteins-for-multi-tasks” NE in terms of screening throughput and efficiency, thus often failing to meet the fast-growing demands for biocatalysts with desired properties. In this study, we design a novel “multi-enzymes-for-multi-substrates” (MEMS) DE model and establish the proof-of-concept by running a NE-mimicking and higher-throughput screening on the basis of “two-P450s-against-seven-substrates” (2P×7S) in one pot. With the multiplied throughput and improved hit rate, we witness a series of convergent evolution events of the two archetypal cytochrome P450 enzymes (P450 BM3 and P450cam) in laboratory. It is anticipated that the new strategy of MEMS DE will find broader application for a larger repertoire of enzymes in the future. Furthermore, structural and substrate docking analysis of the two functionally convergent P450 variants provide important insights into how distinct P450 active-sites can reach a common catalytic goal.
Similar content being viewed by others
References
Ahalawat, N., and Mondal, J. (2018). Mapping the substrate recognition pathway in cytochrome P450. J Am Chem Soc 140, 17743–17752.
Alcalde, M., Farinas, E.T., and Arnold, F.H. (2004). Colorimetric high-throughput assay for alkene epoxidation catalyzed by cytochrome P450 BM-3 variant 139–3. J Biomol Screen 9, 141–146.
Arnold, F.H., Wintrode, P.L., Miyazaki, K., and Gershenson, A. (2001). How enzymes adapt: lessons from directed evolution. Trends Biochem Sci 26, 100–106.
Bell, S.G., Chen, X., Xu, F., Rao, Z., and Wong, L.L. (2003a). Engineering substrate recognition in catalysis by cytochrome P450cam. Biochem Soc Trans 31, 558–562.
Bell, S.G., Chen, X., Sowden, R.J., Xu, F., Williams, J.N., Wong, L.L., and Rao, Z. (2003b). Molecular Recognition in (+)-α-pinene oxidation by cytochrome P450cam. J Am Chem Soc 125, 705–714.
Boddupalli, S.S., Estabrook, R.W., and Peterson, J.A. (1990). Fatty acid monooxygenation by cytochrome P-450BM-3. J Biol Chem 265, 4233–4239.
Brandenberg, O.F., Chen, K., and Arnold, F.H. (2019). Directed evolution of a cytochrome P450 carbene transferase for selective functionalization of cyclic compounds. J Am Chem Soc 141, 8989–8995.
Chen, Z.H., Zhang, S.X., Long, N., Lin, L.S., Chen, T., Zhang, F.P., Lv, X. Q., Ye, P.Z., Li, N., and Zhang, K.Z. (2016). An improved substrate cocktail for assessing direct inhibition and time-dependent inhibition of multiple cytochrome P450s. Acta Pharmacol Sin 37, 708–718.
Du, L., Dong, S., Zhang, X., Jiang, C., Chen, J., Yao, L., Wang, X., Wan, X., Liu, X., Wang, X., et al. (2017). Selective oxidation of aliphatic C-H bonds in alkylphenols by a chemomimetic biocatalytic system. Proc Natl Acad Sci USA 114, E5129–E5137.
Egami, H., Oguma, T., and Katsuki, T. (2010). Oxidation catalysis of Nb (salan) complexes: asymmetric epoxidation of allylic alcohols using aqueous hydrogen peroxide as an oxidant. J Am Chem Soc 132, 5886–5895.
Follmer, A.H., Mahomed, M., Goodin, D.B., and Poulos, T.L. (2018). Substrate-dependent allosteric regulation in cytochrome P450cam (CYP101A1). J Am Chem Soc 140, 16222–16228.
Frija, L.M.T., Frade, R.F.M., and Afonso, C.A.M. (2011). Isolation, chemical, and biotransformation routes of labdane-type diterpenes. Chem Rev 111, 4418–4452.
García-Granados, A., Martínez, A., Quirós, R., and Extremera, A.L. (1999). Chemical-microbiological semisynthesis of enantio-Ambrox® derivatives. Tetrahedron 55, 8567–8578.
Glieder, A., and Meinhold, P. (2003). High-throughput screens based on NAD(P)H depletion. In: Arnold, F.H., and Georgiou, G., eds. Directed Enzyme Evolution. Methods in Molecular Biology™. New York: Humana Press. 157–170.
Guengerich, F.P., Martin, M.V., Sohl, C.D., and Cheng, Q. (2009). Measurement of cytochrome P450 and NADPH-cytochrome P450 reductase. Nat Protoc 4, 1245–1251.
Gunsalus, I.C., and Wagner, G.C. (1978). Bacterial P-450cam methylene monooxygenase components: cytochrome m, putidaredoxin, and putidaredoxin reductase. In: Methods in Enzymology. New York: Academic Press. 166–188.
Haines, D.C., Tomchick, D.R., Machius, M., and Peterson, J.A. (2001). Pivotal role of water in the mechanism of P450BM-3. Biochemistry 40, 13456–13465.
Jung, S.T., Lauchli, R., and Arnold, F.H. (2011). Cytochrome P450: taming a wild type enzyme. Curr Opin Biotech 22, 809–817.
Li, Z., Jiang, Y., Guengerich, F.P., Ma, L., Li, S., and Zhang, W. (2020). Engineering cytochrome P450 enzyme systems for biomedical and biotechnological applications. J Biol Chem 295, 833–849.
Manchester, J.I., and Ornstein, R.L. (1996). Rational approach to improving reductive catalysis by cytochrome P450cam. Biochimie 78, 714–722.
Markel, U., Essani, K.D., Besirlioglu, V., Schiffels, J., Streit, W.R., and Schwaneberg, U. (2020). Advances in ultrahigh-throughput screening for directed enzyme evolution. Chem Soc Rev 49, 233–262.
Morlock, L.K., Böttcher, D., and Bornscheuer, U.T. (2018). Simultaneous detection of NADPH consumption and H2O2 production using the Ampliflu™ Red assay for screening of P450 activities and uncoupling. Appl Microbiol Biotechnol 102, 985–994.
Morris, G.M., Huey, R., Lindstrom, W., Sanner, M.F., Belew, R.K., Goodsell, D.S., and Olson, A.J. (2009). AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J Comput Chem 30, 2785–2791.
Myers, W.K., Lee, Y.T., Britt, R.D., and Goodin, D.B. (2013). The conformation of P450cam in complex with putidaredoxin is dependent on oxidation state. J Am Chem Soc 135, 11732–11735.
Narhi, L.O., and Fulco, A.J. (1987). Identification and characterization of two functional domains in cytochrome P-450BM-3, a catalytically self-sufficient monooxygenase induced by barbiturates in Bacillus megaterium. J Biol Chem 262, 6683–6690.
Ortiz de Montellano, P.R. (2015). Cytochrome P450: Structure, Mechanism, and Biochemistry. Fourth ed. Switzerland: Springer International Publishing.
Poulos, T.L., Finzel, B.C., and Howard, A.J. (1987). High-resolution crystal structure of cytochrome P450cam. J Mol Biol 195, 687–700.
Prier, C.K., Zhang, R.K., Buller, A.R., Brinkmann-Chen, S., and Arnold, F. H. (2017). Enantioselective, intermolecular benzylic C–H amination catalysed by an engineered iron-haem enzyme. Nat Chem 9, 629–634.
Qi, F., Lei, C., Li, F., Zhang, X., Wang, J., Zhang, W., Fan, Z., Li, W., Tang, G.L., Xiao, Y., et al. (2018). Deciphering the late steps of rifamycin biosynthesis. Nat Commun 9, 2342.
Reetz, M.T., and Carballeira, J.D. (2007). Iterative saturation mutagenesis (ISM) for rapid directed evolution of functional enzymes. Nat Protoc 2, 891–903.
Reetz, M.T., Kahakeaw, D., and Lohmer, R. (2008). Addressing the numbers problem in directed evolution. Chembiochem 9, 1797–1804.
Romero, P.A., and Arnold, F.H. (2009). Exploring protein fitness landscapes by directed evolution. Nat Rev Mol Cell Biol 10, 866–876.
Sakurai, K., Shimada, H., Hayashi, T., and Tsukihara, T. (2009). Substrate binding induces structural changes in cytochrome P450cam. Acta Crystlogr F Struct Biol Cryst Commun 65, 80–83.
Schalk, M., Pastore, L., Mirata, M.A., Khim, S., Schouwey, M., Deguerry, F., Pineda, V., Rocci, L., and Daviet, L. (2012). Toward a biosynthetic route to sclareol and amber odorants. J Am Chem Soc 134, 18900–18903.
Sherman, D.H., Li, S., Yermalitskaya, L.V., Kim, Y., Smith, J.A., Waterman, M.R., and Podust, L.M. (2006). The structural basis for substrate anchoring, active site selectivity, and product formation by P450 PikC from Streptomyces venezuelae. J Biol Chem 281, 26289–26297.
Tee, K.L., and Schwaneberg, U. (2006). A screening system for the directed evolution of epoxygenases: importance of position 184 in P450 BM3 for stereoselective styrene epoxidation. Angew Chem Int Ed 45, 5380–5383.
Urban, P., Truan, G., and Pompon, D. (2014). High-throughput functional screening of steroid substrates with wild-type and chimeric P450 enzymes. Biomed Res Int 2014, 1–11.
Wang, B., Li, C., Dubey, K.D., and Shaik, S. (2015). Quantum mechanical/molecular mechanical calculated reactivity networks reveal how cytochrome P450cam and its T252A mutant select their oxidation pathways. J Am Chem Soc 137, 7379–7390.
Whitehouse, C.J.C., Bell, S.G., and Wong, L.L. (2012). P450BM3 (CYP102A1): connecting the dots. Chem Soc Rev 41, 1218–1260.
Wong, T.S., Wu, N., Roccatano, D., Zacharias, M., and Schwaneberg, U. (2005). Sensitive assay for laboratory evolution of hydroxylases toward aromatic and heterocyclic compounds. J Biomol Screen 10, 246–252.
Xu, H., Liang, W., Ning, L., Jiang, Y., Yang, W., Wang, C., Qi, F., Ma, L., Du, L., Fourage, L., et al. (2020). Directed evolution of P450 fatty acid decarboxylases via high-throughput screening towards improved catalytic Activity. Chemcatchem 12, 80–84.
Xue, Y., Wilson, D., Zhao, L., Liu, H., and Sherman, D.H. (1998). Hydroxylation of macrolactones YC-17 and narbomycin is mediated by the pikC-encoded cytochrome P450 in Streptomyces venezuelae. Chem Biol 5, 661–667.
Zeymer, C., and Hilvert, D. (2018). Directed evolution of protein catalysts. Annu Rev Biochem 87, 131–157.
Zhang, K., El Damaty, S., and Fasan, R. (2011). P450 fingerprinting method for rapid discovery of terpene hydroxylating P450 catalysts with diversified regioselectivity. J Am Chem Soc 133, 3242–3245.
Zhang, K., Shafer, B.M., Demars II, M.D., Stern, H.A., and Fasan, R. (2012). Controlled oxidation of remote sp3 C-H bonds in artemisinin via P450 catalysts with fine-tuned regio- and stereoselectivity. J Am Chem Soc 134, 18695–18704.
Zhang, R.K., Chen, K., Huang, X., Wohlschlager, L., Renata, H., and Arnold, F.H. (2019). Enzymatic assembly of carbon-carbon bonds via iron-catalysed sp3 C-H functionalization. Nature 565, 67–72.
Zhang, W., Liu, Y., Yan, J., Cao, S., Bai, F., Yang, Y., Huang, S., Yao, L., Anzai, Y., Kato, F., et al. (2014). New reactions and products resulting from alternative interactions between the P450 enzyme and redox partners. J Am Chem Soc 136, 3640–3646.
Zhang, W., Du, L., Li, F., Zhang, X., Qu, Z., Han, L., Li, Z., Sun, J., Qi, F., Yao, Q., et al. (2018). Mechanistic insights into interactions between bacterial class I P450 enzymes and redox partners. ACS Catal 8, 9992–10003.
Zhou, H., Wang, B., Wang, F., Yu, X., Ma, L., Li, A., and Reetz, M.T. (2019). Chemo- and regioselective dihydroxylation of benzene to hydroquinone enabled by engineered cytochrome P450 monooxygenase. Angew Chem Int Ed 58, 764–768.
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2019YFA0706900), the National Natural Science Foundation of China (32025001, 31872729, 31600045, 32071266, 31800664, 82022066, and 31800041), the Natural Science Foundation of Shandong Province, China (ZR2019ZD20, ZR2016CQ05, and ZR2019QC009), the Laboratory for Marine Drugs and Bioproducts of Pilot National Laboratory for Marine Science and Technology (Qingdao) (LMDBKF-2019-01), the Tianjin Synthetic Biotechnology Innovation Capability Improvement Project (TSBICIP-KJGG-001), the State Key Laboratory of Bio-organic and Natural Products Chemistry (SKLBNPC18242), the Fundamental Research Funds of Shandong University (2019GN030 and 2019GN033), and the Foundation of Qilu University of Technology of Cultivating Subject for Biology and Biochemistry (No. 202014). We would like to thank Zhifeng Li, Jing Zhu and Jingyao Qu from the State Key laboratory of Microbial Technology of Shandong University for help and guidance in GC-MS and HRMS analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Compliance and ethics The author(s) declare that they have no conflict of interest.
Supporting Information
11427_2021_1994_MOESM1_ESM.pdf
Development of MEMS directed evolution strategy for multiplied throughput and convergent evolution of cytochrome P450 enzymes
Rights and permissions
About this article
Cite this article
Ma, L., Li, F., Zhang, X. et al. Development of MEMS directed evolution strategy for multiplied throughput and convergent evolution of cytochrome P450 enzymes. Sci. China Life Sci. 65, 550–560 (2022). https://doi.org/10.1007/s11427-021-1994-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11427-021-1994-1