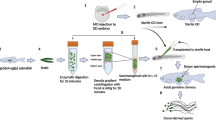
Abstract
The surrogate reproduction technique, such as inter-specific spermatogonial stem cells (SSCs) transplantation (SSCT), provides a powerful tool for production of gametes derived from endangered species or those with desirable traits. However, generation of genome-edited gametes from a different species or production of gametes from a phylogenetically distant species such as from a different subfamily, by SSCT, has not succeeded. Here, using two small cyprinid fishes from different subfamilies, Chinese rare minnow (gobiocypris rarus, for brief: Gr) and zebrafish (danio rerio), we successfully obtained Gr-derived genome-edited sperm in zebrafish by an optimized SSCT procedure. The transplanted Gr SSCs supported the host gonadal development and underwent normal spermatogenesis, resulting in a reconstructed fertile testis containing Gr spermatids and zebrafish testicular somatic cells. Interestingly, the surrogate spermatozoa resembled those of host zebrafish but not donor Gr in morphology and swimming behavior. When pou5f3 and chd knockout Gr SSCs were transplanted, Gr-derived genome-edited sperm was successfully produced in zebrafish. This is the first report demonstrating surrogate production of gametes from a different subfamily by SSCT, and surrogate production of genome-edited gametes from another species as well. This method is feasible to be applied to future breeding of commercial fish and livestock.
Similar content being viewed by others
References
Anders, S., and Huber, W. (2010). Differential expression analysis for sequence count data. Genome Biol 11, R106.
Beer, R.L., and Draper, B.W. (2013). Nanos3 maintains germline stem cells and expression of the conserved germline stem cell gene nanos2 in the zebrafish ovary. Dev Biol 374, 308–318.
Bellaiche, J., Lareyre, J.J., Cauty, C., Yano, A., Allemand, I., and Le Gac, F. (2014). Spermatogonial stem cell quest: nanos2, marker of a subpopulation of undifferentiated A spermatogonia in trout testis. Biol Reprod 90, 79.
Brinster, R.L. (2002). Germline stem cell transplantation and transgenesis. Science 296, 2174–2176.
Burgess, S., Reim, G., Chen, W., Hopkins, N., and Brand, M. (2002). The zebrafish spiel-ohne-grenzen (spg) gene encodes the POU domain protein Pou2 related to mammalian Oct4 and is essential for formation of the midbrain and hindbrain, and for pre-gastrula morphogenesis. Development 129, 905–916.
Chang, N., Sun, C., Gao, L., Zhu, D., Xu, X., Zhu, X., Xiong, J.W., and Xi, J.J. (2013). Genome editing with RNA-guided cas9 nuclease in zebrafish embryos. Cell Res 23, 465–472.
Chen, Q., Yan, W., and Duan, E. (2016). Epigenetic inheritance of acquired traits through sperm RNAs and sperm RNA modifications. Nat Rev Genet 17, 733–743.
Cinalli, R.M., Rangan, P., and Lehmann, R. (2008). Germ cells are forever. Cell 132, 559–562.
Ciruna, B., Weidinger, G., Knaut, H., Thisse, B., Thisse, C., Raz, E., and Schier, A.F. (2002). Production of maternal-zygotic mutant zebrafish by germ-line replacement. Proc Natl Acad Sci USA 99, 14919–14924.
Cossetti, C., Lugini, L., Astrologo, L., Saggio, I., Fais, S., and Spadafora, C. (2014). Soma-to-germline transmission of RNA in mice xenografted with human tumour cells: possible transport by exosomes. PLoS ONE 9, e101629.
Goto, R., and Saito, T. (2019). A state-of-the-art review of surrogate propagation in fish. Theriogenology 133, 216–227.
Gratacap, R.L., Wargelius, A., Edvardsen, R.B., and Houston, R.D. (2019). Potential of genome editing to improve aquaculture breeding and production. Trends Genet 35, 672–684.
Hammerschmidt, M., Pelegri, F., Mullins, M.C., Kane, D.A., van Eeden, F. J., Granato, M., Brand, M., Furutani-Seiki, M., Haffter, P., Heisenberg, C.P., et al. (1996). Dino and mercedes, two genes regulating dorsal development in the zebrafish embryo. Development 123, 95–102.
Houston, R.D., Bean, T.P., Macqueen, D.J., Gundappa, M.K., Jin, Y.H., Jenkins, T.L., Selly, S.L.C., Martin, S.A.M., Stevens, J.R., Santos, E.M., et al. (2020). Harnessing genomics to fast-track genetic improvement in aquaculture. Nat Rev Genet 21, 389–409.
Huang, D.W., Sherman, B.T., and Lempicki, R.A. (2009). Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4, 44–57.
Ivell, R., and Bathgate, R.A.D. (2002). Reproductive biology of the relaxin-like factor (RLF/INSL3). Biol Reprod 67, 699–705.
Iwasaki-Takahashi, Y., Shikina, S., Watanabe, M., Banba, A., Yagisawa, M., Takahashi, K., Fujihara, R., Okabe, T., Valdez Jr, D.M., Yamauchi, A., et al. (2020). Production of functional eggs and sperm from in vitro-expanded type A spermatogonia in rainbow trout. Commun Biol 3, 308.
Jin, Y.H., Robledo, D., Hickey, J.M., McGrew, M.J., and Houston, R.D. (2021). Surrogate broodstock to enhance biotechnology research and applications in aquaculture. Biotechnol Adv 49, 107756.
Kimmel, C.B., Ballard, W.W., Kimmel, S.R., Ullmann, B., and Schilling, T. F. (1995). Stages of embryonic development of the zebrafish. Dev Dyn 203, 253–310.
Kubota, H., and Brinster, R.L. (2018). Spermatogonial stem cells. Biol Reprod 99, 52–74.
Lauter, G., Söll, I., and Hauptmann, G. (2011). Multicolor fluorescent in situ hybridization to define abutting and overlapping gene expression in the embryonic zebrafish brain. Neural Dev 6, 10.
Leal, M.C., Cardoso, E.R., Nóbrega, R.H., Batlouni, S.R., Bogerd, J., França, L.R., and Schulz, R.W. (2009). Histological and stereological evaluation of zebrafish (Danio rerio) spermatogenesis with an emphasis on spermatogonial generations. Biol Reprod 81, 177–187.
Lehmann, R. (2012). Germline stem cells: Origin and destiny. Cell Stem Cell 10, 729–739.
Li, M., Hong, N., Xu, H., Song, J., and Hong, Y. (2016). Germline replacement by blastula cell transplantation in the fish medaka. Sci Rep 6, 29658.
Li, Q., Fujii, W., Naito, K., and Yoshizaki, G. (2017). Application of dead end-knockout zebrafish as recipients of germ cell transplantation. Mol Reprod Dev 84, 1100–1111.
Li, X., Zhang, F., Wu, N., Ye, D., Wang, Y., Zhang, X., Sun, Y., and Zhang, Y.A. (2020). A critical role of foxp3a-positive regulatory T cells in maintaining immune homeostasis in zebrafish testis development. J Genet Genomics 47, 547–561.
Liang, X., and Zha, J. (2016). Toxicogenomic applications of chinese rare minnow (Gobiocypris rarus) in aquatic toxicology. Comp Biochem Physiol Part D-Genomics Proteomics 19, 174–180.
Lubzens, E., Young, G., Bobe, J., and Cerdà, J. (2010). Oogenesis in teleosts: How fish eggs are formed. Gen Comp Endocrinol 165, 367–389.
Luo, S., Jin, S., Su, L., and Wang, J. (2017). Effect of water temperature on reproductive performance and offspring quality of rare minnow, Gobiocypris rarus. J Thermal Biol 67, 59–66.
Morita, T., Kumakura, N., Morishima, K., Mitsuboshi, T., Ishida, M., Hara, T., Kudo, S., Miwa, M., Ihara, S., Higuchi, K., et al. (2012). Production of donor-derived offspring by allogeneic transplantation of spermatogonia in the yellowtail (Seriola quinqueradiata). Biol Reprod 86.
Nasevicius, A., and Ekker, S.C. (2000). Effective targeted gene ‘knockdown’ in zebrafish. Nat Genet 26, 216–220.
Nóbrega, R.H., Greebe, C.D., van de Kant, H., Bogerd, J., de França, L.R., and Schulz, R.W. (2010). Spermatogonial stem cell niche and spermatogonial stem cell transplantation in zebrafish. PLoS ONE 5, e12808.
Octavera, A., and Yoshizaki, G. (2019). Production of donor-derived offspring by allogeneic transplantation of spermatogonia in Chinese rosy bitterling. Biol Reprod 100, 1108–1117.
Ohta, H., Wakayama, T., and Nishimune, Y. (2004). Commitment of fetal male germ cells to spermatogonial stem cells during mouse embryonic development. Biol Reprod 70, 1286–1291.
Okutsu, T., Suzuki, K., Takeuchi, Y., Takeuchi, T., and Yoshizaki, G. (2006). Testicular germ cells can colonize sexually undifferentiated embryonic gonad and produce functional eggs in fish. Proc Natl Acad Sci USA 103, 2725–2729.
Okutsu, T., Shikina, S., Kanno, M., Takeuchi, Y., and Yoshizaki, G. (2007). Production of trout offspring from triploid salmon parents. Science 317, 1517.
Pérez-Cadahía B., Drobic, B., and Davie, J.R. (2009). H3 phosphorylation: dual role in mitosis and interphase. Biochem Cell Biol 87, 695–709.
Rodríguez-Marí, A., Yan, Y.L., Bremiller, R.A., Wilson, C., Cañestro, C., and Postlethwait, J.H. (2005). Characterization and expression pattern of zebrafish anti-Müllerian hormone (amh) relative to sox9a, sox9b, and cyp19a1a, during gonad development. Gene Expr Patterns 5, 655–667.
Saito, T., Fujimoto, T., Maegawa, S., Inoue, K., Tanaka, M., Arai, K., and Yamaha, E. (2006). Visualization of primordial germ cells in vivo using GFP-nos1 3′UTR mRNA. Int J Dev Biol 50, 691–699.
Sawatari, E., Shikina, S., Takeuchi, T., and Yoshizaki, G. (2007). A novel transforming growth factor-β superfamily member expressed in gonadal somatic cells enhances primordial germ cell and spermatogonial proliferation in rainbow trout (Oncorhynchus mykiss). Dev Biol 301, 266–275.
Schulte-Merker, S., Lee, K.J., McMahon, A.P., and Hammerschmidt, M. (1997). The zebrafish organizer requires chordino. Nature 387, 862–863.
Schulz, R.W., de França, L.R., Lareyre, J.J., Le Gac, F., LeGac, F., Chiarini-Garcia, H., Nobrega, R.H., and Miura, T. (2010). Spermatogenesis in fish. Gen Comp Endocrinol 165, 390–411.
Slanchev, K., Stebler, J., de la Cueva-Méndez, G., and Raz, E. (2005). Development without germ cells: The role of the germ line in zebrafish sex differentiation. Proc Natl Acad Sci USA 102, 4074–4079.
Sun, Y., and Zhu, Z. (2019). Designing future farmed fishes using genome editing. Sci China Life Sci 62, 420–422.
Sun, Y., Zhang, B., Luo, L., Shi, D.L., Wang, H., Cui, Z., Huang, H., Cao, Y., Shu, X., Zhang, W., et al. (2020). Systematic genome editing of the genes on zebrafish chromosome 1 by CRISPR/Cas9. Genome Res 30, 118–126.
Tajima, A., Naito, M., Yasuda, Y., and Kuwana, T. (1993). Production of germ line chimera by transfer of primordial germ cells in the domestic chicken. Theriogenology 40, 509–519.
Takeuchi, Y., Yoshizaki, G., and Takeuchi, T. (2003). Generation of live fry from intraperitoneally transplanted primordial germ cells in rainbow trout. Biol Reprod 69, 1142–1149.
Takeuchi, Y., Yoshizaki, G., and Takeuchi, T. (2004). Surrogate broodstock produces salmonids. Nature 430, 629–630.
Thisse, B., Heyer, V., Lux, A., Alunni, V., Degrave, A., Seiliez, I., Kirchner, J., Parkhill, J.P., Thisse, C. (2004). Spatial and temporal expression of the zebrafish genome by large-scale in situ hybridization screening. Methods Cell Biol 77, 505–519.
Weidinger, G., Stebler, J., Slanchev, K., Dumstrei, K., Wise, C., Lovell-Badge, R., Thisse, C., Thisse, B., and Raz, E. (2003). Dead end, a novel vertebrate germ plasm component, is required for zebrafish primordial germ cell migration and survival. Curr Biol 13, 1429–1434.
Wong, T.T., Saito, T., Crodian, J., and Collodi, P. (2011). Zebrafish germline chimeras produced by transplantation of ovarian germ cells into sterile host larvae. Biol Reprod 84, 1190–1197.
Ye, D., Zhu, Z.Y., and Sun, Y.H. (2015). Fish genome manipulation and directional breeding. Sci China Life Sci 58, 170–177.
Ye, D., Zhu, L., Zhang, Q., Xiong, F., Wang, H., Wang, X., He, M., Zhu, Z., and Sun, Y. (2019). Abundance of early embryonic primordial germ cells promotes zebrafish female differentiation as revealed by lifetime labeling of germline. Mar Biotechnol 21, 217–228.
Yoshikawa, H., Ino, Y., Shigenaga, K., Katayama, T., Kuroyanagi, M., and Yoshiura, Y. (2018). Production of tiger puffer Takifugu rubripes from cryopreserved testicular germ cells using surrogate broodstock technology. Aquaculture 493, 302–313.
Yoshikawa, H., Morishima, K., Fujimoto, T., Saito, T., Kobayashi, T., Yamaha, E., and Arai, K. (2009). Chromosome doubling in early spermatogonia produces diploid spermatozoa in a natural clonal fish. Biol Reprod 80, 973–979.
Yoshikawa, H., Ino, Y., Kishimoto, K., Koyakumaru, H., Saito, T., Kinoshita, M., and Yoshiura, Y. (2020). Induction of germ cell-deficiency in grass puffer by dead end 1 gene knockdown for use as a recipient in surrogate production of tiger puffer. Aquaculture 526, 735385.
Yoshikawa, H., Takeuchi, Y., Ino, Y., Wang, J., Iwata, G., Kabeya, N., Yazawa, R., and Yoshizaki, G. (2017). Efficient production of donor-derived gametes from triploid recipients following intra-peritoneal germ cell transplantation into a marine teleost, nibe croaker (Nibea mitsukurii). Aquaculture 478, 35–47.
Yuan, L., Liu, J.G., Zhao, J., Brundell, E., Daneholt, B., and Höög, C. (2000). The murine scp3 gene is required for synaptonemal complex assembly, chromosome synapsis, and male fertility. Mol Cell 5, 73–83.
Zhang, F. H., Wang, H.P., Huang, S. Y., Xiong, F., Zhu, Z. Y., and Sun, Y.H. (2016). A comparison of the knockout efficiencies of two codon-optimized Cas9 coding sequences in zebrafish embryos. Hereditas 38, 144–154.
Zhang, F., Li, X., He, M., Ye, D., Xiong, F., Amin, G., Zhu, Z., and Sun, Y. (2020). Efficient generation of zebrafish maternal-zygotic mutants through transplantation of ectopically induced and Cas9/gRNA targeted primordial germ cells. J Genet Genomics 47, 37–47.
Acknowledgements
We sincerely thank Ms Ming Li at Institute of Hydrobiology, CAS for providing Gobiocypris rarus embryos and we thank Kuoyu Li at the China Zebrafish Resource Center for zebrafish and Gobiocypris rarus rearing. We also thank Ms Zhixian Qiao, Fang Zhou and Yuan Xiao at analytical and testing center of Institute of Hydrobiology, CAS for providing technical support in RNA-seq, confocal microscope, SEM and TEM analysis. This work was supported by the National Natural Science Foundation of China (32025037 and 31721005), the National Key R&D Project of China (2018YFA0801000 and 2018YFD0901205), Chinese Academy of Sciences (XDA24010108), and State Key Laboratory of Freshwater Ecology and Biotechnology (2019FBZ05).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Zhang, F., Hao, Y., Li, X. et al. Surrogate production of genome-edited sperm from a different subfamily by spermatogonial stem cell transplantation. Sci. China Life Sci. 65, 969–987 (2022). https://doi.org/10.1007/s11427-021-1989-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11427-021-1989-9