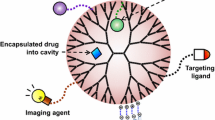
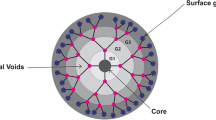
Abstract
Peptide dendrimers are attractive synthetic polymers and have been widely used as a new generation of biomaterials in recent years. Peptide dendrimers, as well as general dendrimers, may be synthesized to reach nano sizes, and display well-defined architectures, highly-branched structures, high density of functional terminal groups, and controllable molecular weights. On the other hand, peptide dendrimers have properties similar to proteins and some special characteristics, such as good biocompatibility, water solubility and resistance to proteolytic digestion. Due to these advantages, peptide dendrimers have received considerable attention in biomedicine. This review focuses on the development of peptide dendrimers with emphasis on their applications both in diagnostics and in therapy.
Similar content being viewed by others
References
Bosman AW, Janssen HM, Meijer EW. About dendrimers: Structure, physical properties, and applications. Chem Rev, 1999, 99: 1665–1688
Flory PJ. Molecular size distribution in three dimensional polymers. VI. Branched polymers containing A-R-Bf-1 type units. J Am Chem Soc, 1952, 74: 2718–2723
Buhleier E, Wehner W, Vögtle F. Cascade and nonskid-chain-like syntheses of molecular cavity topologies. Synthesis, 1978, 155–158
Tomalia DA, Baker H, Dewald J, Hall M, Kallos G, Martin S, Roeck J, Ryder J, Smith P. A new class of polymers: Starburst-dendritic macromolecules. Polym J, 1985, 17: 117–132
Newkome GR, Yao ZQ, Baker GR, Gupta VK. Micelles part 1, cascade molecules: A new approach to micelles. A [27]-arborol. J Org Chem, 1985, 50: 2003–2004
Crespo L, Sanclimens G, Pons M, Giralt E, Royo M. Peptide and amide bond-containing dendrimers. Chem Rev, 2005, 105: 1663–1681
Sadler K, Tam JP. Peptide dendrimers: Applications and synthesis. Molecular Biotechnol. 2002, 90: 195–229
Kensinger RD, Catalone BJ, Krebs FC, Wigdahl B, Schengrund CL. Novel polysulfated galactose-derivatized dendrimers as binding antagonists of human immunodeficiency virus type 1 infection. Antimic Agents Chemother, 2004 48: 1614–1623
Kinberger GA, Cai WB, Goodman M. Collagen mimetic dendrimers. J Am Chem Soc, 2002, 124: 15162–15163
Zeng F, Zimmerman SC. Dendrimers in supramolecular chemistry: From molecular recognition to self-assembly, Chem Rev, 1997, 97: 1681–1712
Kaneshiro TL, Wang XL, Lu ZR. Synthesis, characterization and gene delivery of poly-l-lysine octa(3-aminopropyl) silsesquioxane dendrimers: Nanoglobular drug carriers with precisely defined molecular architectures. Mol Pharmaceutics, 2007, 4: 759–768
Dykes GM, Brierley LJ, Smith DK, McGrail PT, Seeley GJ. Supramolecular solubilisation of hydrophilic dyes by using individual dendritic branches, Chem Eur J, 2001, 7: 4730–4739
Choi JS, Lee EJ, Choi YH, Jeong YJ, Park JS. Poly(ethyleneglycol)-block-poly(l-lysine) dendrimer: novel linear polymer/dendrimer block copolymer forming a spherical water-soluble polyionic complex with DNA. Bioconjugate Chem, 1999, 10: 62–65
Bodanzky M, Bodanzky A, Bailey P. The Practice of Peptide Synthesis. New York: Springer, 1984
Sakakibara S. Synthesis of large peptides in solution. Biopolymers, 1995, 37: 17–28
Sakakibara S. Chemical synthesis of proteins in solution. Biopolymers, 1999, 51: 279–296
Kates SA, Albericio F. Solid-Phase Synthesis. A Practical Guide. New York: Marcel Dekker, 2000
Merrifield RB. Solid phase peptide synthesis I. The synthesis of a tetrapeptide. J Am Chem Soc, 1963, 85: 2149–2154
Zeng ST, Yang Y, Yuan XB, Chang J. Development of synthesis of peptide dendrimer (in Chinese). Chin Polym Bull, 2006, 10: 1–9
Tian SL, Cai MS. Protocol method in solid phase peptide synthesis (in Chinese). Chemistry, 1992, 2: 17–22
Crespo L, Sanclimens G, Montaner B, Pérez-Tomás R, Royo M, Pons M, Albericio F, Giralt E. Peptide dendrimers based on polyproline helices. J Am Chem Soc, 2002, 124: 8876–8883
Sanclimens G, Crespo L, Pons M, Giralt E, Albericio F, Royo M. Saturated resins or stress of the resin. Tetrahedron Lett, 2003, 44: 1751–1754
Denkewalter RG, Kole J, Lukasavage WJ. Macromolecular highly branched homogeneous compound based on lysine units. US Patent 4289872, 1981-09-16
Ohsaki M, Okuda T, Wada A, Hirayama T, Niidome T, Aoyagi H. In vitro gene transfection using dendritic poly(l-lysine). Biconjugate Chem, 2002, 13: 510–517
He J, Ma Y, Zhao YF. Synthesis and applications of peptide dendrimer (in Chinese). Prog Chem, 2005, 17: 468–476
Tam JP, Xu J, Eom KD. Methods and strategies of peptide ligation. Biopolymers, 2001, 60: 194–209
Medina SH, El-Sayed MEH. Dendrimers as carriers for delivery of chemotherapeutic agents. Chem Rev, 2009, 109: 3141–3157
Wu P, Feldman AK, Nugent AK, Hawker CJ, Scheel A, Voit B, Pyun J, Fréchet JMJ, Sharpless KB, Fokin VV. Efficiency and fidelity in a click-chemistry route to triazole dendrimers by the copper(I)-catalyzed ligation of azides and alkynes. Angew Chem Int Ed, 2004, 43: 3928–3932
Wu P, Malkochb M, Huntb JN, Vestbergb R, Kaltgrada E, Finn MG, Fokin VV, Sharpless KB, Hawker CJ. Multivalent bifunctional dendrimers prepared by click chemistry. Chem Commun, 2005: 5775–5777
Kolb HC, Finn MG, Sharpless KB. Click chemistry: Diverse chemical function from a few good reactions. Angew Chem Int Ed, 2001, 40: 2004–2021
Grayson SM, Fréchet JMJ. Convergent dendrons and dendrimers: from synthesis to applications. Chem Rev, 2001, 101: 3819–3968
Lee JW, Kim JH, Kim HL, Han SC, Kim JH, Shin WS, Jin SH. Synthesis of symmetrical and unsymmetrical PAMAM dendrimers by fusion between azide and alkyne functionalized PAMAM dendrons. Bioconjugate Chem, 2007, 18: 579–584
Lee JW, Kim JH, Kim BK, Kim JH, Shin WS, Jin SH. Convergent synthesis of PAMAM dendrimers using click chemistry of azidefunctionalized PAMAM dendrons. Tetrahedron, 2006, 62: 9193–9200
Yim CB, Boerman OC, Visser MD, Jong MD, Dechesne AC, Rijkers DTS, Liskamp RMJ. Versatile conjugation of octreotide to dendrimers by cycloaddition “click” chemistry to yield high-affinity multivalent cyclic peptide dendrimers. Bioconjugate Chem, 2009, 20: 1323–1331
Kuijpers BHM, Groothuys S, Soede AC, Laverman P, Boerman OC, Delft FLV, Rutjes FPJT. Preparation and evaluation of glycosylated arginine-glycine-aspartate (RGD) derivatives for integrin targeting. Bioconjugate Chem, 2007, 18: 1847–1854
Ellen MM, van Brander DB, Meijer EW. Poly(propylene imine) dendrimers: Large-scale synthesis by hetereogeneously catalyzed hydrogenations. Angew Chem Int Ed, 1993, 32: 1308–1311
Hawker C, Fréchet JMJ. A new convergent approach to monodisperse dendritic macromolecules. J Chem Soc Chem Commun, 1990, (15): 1010–1013
Hawker CJ, Fréchet JMJ. Preparation of polymers with controlled molecular architecture A new convergent approach to dendritic macromolecules. J Am Chem Soc, 1990, 112: 7638–7647
Hawker CJ, Wooley KL, Fréchet JMJ. Unimolecular micelles and globular amphiphiles:dendritic macromolecules as novel recyclable solubilization agents. J Chem Soc Perkin Trans 1, 1993, 1287–1297
Kawaguchi T, Walker KL, Wilkins CL, Moore JS. Double exponential dendrimer growth. J Am Chem Soc, 1995, 117: 2159–2165
Kantchev EAB, Chang CC, Chang DK. Direct Fmoc/tert-Bu solid phase synthesis of octamannosyl polylysine dendrimer-peptide conjugates. Biopolymers, 2006, 84: 232–240
Shao J, Tam JP. Unprotected peptides as building blocks for the synthesis of peptide dendrimers with oxime, hydrazone, and thiazolidine linkages. J Am Chem Soc, 1995, 117: 3893–3899
Sasaki T, Kaiser ET. Helichrome: synthesis and enzymic activity of a designed hemeprotein. J Am Chem Soc, 1989, 111: 380–381
Finikova O, Galkin A, Rozhkov V, Cordero M, Hägerhäll C, Vinogradov S. Porphyrin and tetrabenzoporphyrin dendrimers: Tunable membrane-impermeable fluorescent pH nanosensors. J Am Chem Soc, 2003, 125: 4882–4893
Xu M, Tang K, Zhang T, Yu Xuhai. Progress in functional dendrimers. J Funct Poly, 2001, 14: 481–487
Leiding T, Górecki, K, Kjellman T, Vinogradov SA, Hägerhäll C, Årsköld SP. Precise detection of pH inside large unilamellar vesicles using membrane-impermeable dendritic porphyrin-based nanoprobes. Anal Biochem, 2009, 2: 296–305
Choi JS, Joo DH, Kim CH, Kim K, Park JS. Synthesis of a barbell-like triblock copolymer, poly(l-lysine) dendrimer-block-poly (ethyleneglycol)-block-poly(l-lysine) dendrimer, and its self-assembly with plasmid DNA. J Am Chem Soc, 2000, 122: 474–480
Chapman TM, Hillyer GL, Mahan EJ, Shaffer KA. Hydraamphiphiles: Novel linear dendritic block copolymer surfactants. J Am Chem Soc, 1994, 116: 11195–11196
Kress J, Rosner A, Hirsch A. Depsipeptide dendrimers. Chem Eur J, 2000, 6: 247–257
Kim YS, Gil ES, Lowe TL. Synthesis and characterization of thermoresponsive co-biodegradable linear-dendritic copolymers. Macromolecules, 2006, 39: 7805–7811
Kaminskas LM, Boyd BJ, Karellas P, Krippner GY, Lessene R, Kelly B, Porter CJH. The impact of molecular weight and PEG chain length on the systemic pharmacokinetics of PEGylated poly l-lysine dendrimers. Mol Pharmaceutics, 2008, 5: 449–463
Gillies ER, Dy E, Fréchet JMJ, Szoka FC. Biological evaluation of polyester dendrimer: Poly(ethylene oxide) bow-tie hybrids with tunable molecular weight and architecture. Mol Pharm, 2005, 2: 129–138
Florence AT, Sakthivel T, Toth I. Oral uptake and translocation of a polylysine dendrimer with a lipid surface. J Control Release, 2000, 65: 253–259
Wolinsky JB, Grinstaff MW. Therapeutic and diagnostic applications of dendrimers for cancer treatment. Adv Drug Deliv Rev, 2008, 60: 1037–1055
Sashima H, Shigemasa Y, Roy R. Chemical modification of chitosan hyperbranched chitosan-sialic acid dendrimer hybrid with tetraethylene glycol spacer. Macromolecules, 2000, 33: 6913–6915
Niederhafner P, Sebestika J, Jezeka J. Glycopeptide dendrimers. Part I. J Pept Sci, 2008, 14: 2–43
Niederhafner P, Sebestika J, Jezeka J. Glycopeptide dendrimers. Part II. J Pept Sci, 2008, 14: 44–65
Niederhafner P, Sebestika J, Jezeka J. Glycopeptide dendrimers. Part III-a review: Use of glycopeptide dendrimers in immunotherapy and diagnosis of cancer and viral diseases. J Pept Sci, 2008, 14: 556–587
Baigude H, Katsuraya K, Okuyama K, Yachi Y, Sato S, Uryu T. Synthesis of dicarboxylate oligosaccharide multilayer terminal functionality upon poly(lysine) dendrimer scaffolding. J Polym Sci Part A Polym Chem. 2002, 40: 3622–3633
Fu YJ, Nitecki DE, Maltby D, Simon GH, Berejnoi K, Raatschen HJ, Yeh BM, Shames DM, Brasch RC. Dendritic iodinated contrast agents with PEG-cores for CT imaging: Synthesis and preliminary characterization. Bioconjugate Chem, 2006, 17: 1043–1056
Galande AK, Hilderbrand SA, Weissleder R, Tung CH. Enzyme-targeted fluorescent imaging probes on a multiple antigenic peptide core. J Med Chem, 2006, 49: 4715–4720
Hong S, Leroueil PR, Majoros IJ, Orr BG, Baker JR, Holl MMB. The binding avidity of nanoparticle-base multivalent targeted drug delivery platform. Chem Biol, 2007, 14:107–115
Majoros IJ, Thomas TP, Mehta CB, Baker JR. Poly(amidoamine) dendrimer-based multifunctional engineered nanodevice for cancer therapy. J Med Chem, 2005, 48: 5892–5899
Singh P, Gupta U, Asthana A, Jain NK. Folate and folate-PEGPAMAM dendrimers: synthesis, characterization, and targeted anticancer drug delivery potential in tumor bearing mice. Bioconjugate Chem, 2008, 19: 2239–2252
Torchilin VP. Multifunctional nanocarriers. Adv Drug Deliv Rev, 2006, 58: 1532–1555
Sanvicens N, Marco MP. Multifunctional nanoparticles-properties and prospects for use in human medicine. Trends Biotechnol, 2008, 26: 425–433
Bogdanov AA, Weissleder R, Brady TJ. Long-circulating blood pool imaging agents. Adv Drug Deliver Rev, 1995, 16: 335–348
Sachse A, Leike JUD, Thomas S, Wagner SED, Roling GL, Krause W, Brandl M. Biodistribution and computed tomography blood-pool imaging properties of polyethylene glycol-coated iopromide-carrying liposomes. Invest Radiol, 1997, 32: 44–50
Yordanov AT, Lodder AL, Woller EK, Cloninger MJ, Patronas N, Milenic D, Brechbiel MW. Novel iodinated dendritic nanoparticles for computed tomography (CT) imaging. Nano Lett, 2002, 2: 595–599
Krause W, Hackmann-Schlichter N, Maier FK, Müller R. Dendrimers in diagnostics. (in: Top Curr Chem). Heidelberg Springer, 2000, 210: 261–308
Li C, Winnard PT, Takagi T, Artemov D, Bhujwalla ZM. multimodal image-guided enzyme/prodrug cancer therapy. J Am Chem Soc, 2006, 128: 15072–15073
Daldrup-Link HE, Brasch RC. Breast MR imaging contrast medium macromolecular contrast medium. Eur Radiol, 2003, 13: 354–365
Wiener EC, Auteri FP, Chen JW, Brechbiel MW, Gansow OA, Schneider DS, Belford RL, Clarkson RB, Lauterbur PC. Molecular dynamics of ion-chelate complexes attached to dendrimers. J Am Chem Soc, 1996, 118: 7774–7782
Wiener EC, Brechbiel MW, Brothers H, Magin RL, Gansow OA, Tomalia DA, Lauterbur PC. Dendrimer-based metal chelates: A new class of magnetic resonance imaging contrast agents. Magn Reson Med, 1994, 31: 1–8
Kobayashi H, Kawamoto S, Jo SK, Bryant HL, Brechbiel MW, Star RA. Macromolecular MRI contrast agents with small dendrimers: Pharmacokinetic differences between sizes and cores. Bioconjugate Chem, 2003, 14: 388–394
Sato N, Kobayashi H, Hiraga A, Saga T, Togashi K, Konishi J, Brechbiel MW. Pharmacokinetics and enhancement patterns of macromolecular MR contrast agents with various sizes of polyamidoamine dendrimer cores. Magn Reson Med, 2001, 46: 1169–1173
Cloninger MJ. Biological applications of dendrimers. Curr Opin Chem Biol, 2002, 6: 742–748
Luo K. New Generation Biomedical Materials: Peptide Dendrimers and Their Application in Biomedicine (in Chinese). Ph D Dissertation of Sichuan Universtiy, 2009
Fu YJ, Raatschen HJ, Nitecki DE, Wendland MF, Novikov V, Fournier LS, Cyran C, Victor R, Shames DM, Brasch RC. Cascade polymeric MRI contrast media derived from poly(ethylene glycol) cores: Initial syntheses and characterizations. Biomacromolecules, 2007, 8: 1519–1529
Liu M, Guo YM, Wang P, Guo XJ, Yang JL. Characteristics and in vitro imaging study of matrix metalloproteinase-2 targeting activable cell penetrating peptide (in Chinese). National Med J China, 2007, 87(4): 233–239
Boutry S, Burtea C, Laurent S, Toubeau G, Elst LV, Muller RN. Magnetic resonance imaging of inflammation with a specific selectintargeted contrast agent. Magn Reson Med, 2005, 53: 800–807
Wiener EC, Konda S, Shadron A, Brechbiel M, Gansow O. Targeting dendrimer-chelates to tumors and tumor cells expressing the high-affinity folate receptor. Invest Radiol, 1997, 32: 748–754
Konda SD, Aref M, Brechbiel M, Wiener EC. Development of a tumor-targeting MR contrast agent using the high-affinity folate receptor — Work in progress. Invest Radiol, 2000, 35: 50–57
Takahashi M, Hara Y, Aoshima K, Kurihara H, Oshikawa T, Yamashita M. Utilization of dendritic framework as a multivalent ligand: A functionalized gadolinium(III) carrier with glycoside cluster periphery. Tetrahedron Lett, 2000, 41: 8485–8488
Woller EK, Walter ED, Morgan JR, Singel DJ, Cloninger MJ. Altering the strength of lectin binding interactions and controlling the amount of lectin clustering using mannose/hydroxyl-functionalized dendrimers. J Am Chem Soc, 2003, 125: 8820–8826
Wolfenden ML, Cloninger MJ. Mannose/glucose-functionalized dendrimers to investigate the predictable tunability of multivalent interactions. J Am Chem Soc, 2005, 127: 12168–12169
Zanini D, Roy R. Synthesis of new α-thiosialodendrimers and their binding properties to the sialic acid specific lectin from limax flavus. J Am Chem Soc, 1997, 119: 2088–2095
Zanini D, Roy R. Practical synthesis of starburst PAMAM α-thiosialodendrimers for probing multivalent carbohydrate-lectin binding properties. J Org Chem, 1998, 63: 3486–3491
Baal I, Malda H, Synowsky SA, van Dongen JLJ, Hackeng TM, Merkx M, Meijer EW. Multivalent peptide and protein dendrimers using native chemical ligation. Angew Chem Int Ed, 2005, 44: 5052–5057
Rijkers DTS, Esse GW, Merkx R, Brouwer AJ, Jacobs HJF, Pieters RJ, Liskamp RMJ. Efficient microwave-assisted synthesis of multivalent dendrimeric peptides using cycloaddition reaction (click) chemistry. Chem Commun, 2005, 4581–4583
Kluger R, Zhang J. Hemoglobin dendrimers: Functional protein clusters. J Am Chem Soc, 2003, 125: 6070–6071
Wu CC, Brechbiel MW, Kozak RW, Gansow OA. Metal-chelatedendrimer-antibody constructs for use in radioimmunotherapy and imaging. Bioorg Med Chem Lett, 1994, 4: 449–454
Choi YS, Mecke A, Orr BG, Holl MMB, Baker JR. DNA-directed synthesis of generation 7 and 5 PAMAM dendrimer nanoclusters. Nano Lett, 2004, 4: 391–397
Konda SD, Aref M, Wang S, Brechbiel M, Wiener EC. Specific targeting of folate-dendrimer MRI contrast agents to the high affinity folate receptor expressed in ovarian tumor xenografts. Magnetic resonance materials in physics. Biol Med, 2001, 12: 104–113
Konda SD, Wang S, Brechbiel M, Wiener EC. Biodistribution of a Gd-153-folate dendrimer, generation = 4, in mice with folate-receptor positive and negative ovarian tumor xenografts. Invest Radiol, 2002, 37: 199–204
Swanson SD, Kukowska-Latallo JF, Patri AK, Chen CY, Ge S, Cao ZY, Kotlyar A, East AT, Baker JR. Targeted gadolinium-loaded dendrimer nanoparticles for tumor-specific magnetic resonance contrast enhancement. Int J Nanomed, 2008, 3(2): 201–210
Dirksen A, Langereis S, Waal BFM, Genderen MHP, Hackeng TM, Meijer EW. A supramolecular approach to multivalent target-specific MRI contrast agents for angiogenesis. Chem Commun, 2005, 2811–2813
Xu H, Regino CAS, Koyama Y, Hama Y, Gunn AJ, Bernardo M, Kobayashi H, Choyke PL, Brechbiel MW. Preparation and preliminary evaluation of a biotin-targeted, lectin-targeted dendrimer-based probe for dual-modality magnetic resonance and fluorescence imaging. Bioconjugate Chem, 2007, 18: 1474–1482
Anderson SA, Rader RK, Westlin WF, Null C, Jackson D, Lanza GM, Wickline SA, Kotyk JJ. Magnetic resonance contrast enhancement of neovasculature with αvβ3-targeted nanoparticles. Magn Reson Med, 2000, 44: 433–439
Luo K, Liu G, Zhang XW, She WC, He B, Nie Y, Li L, Wu Y, Zhang ZR, Gong QY, Gao FB, Song B, Ai H, Gu ZW. Functional L-lysine dendritic macromolecules as liver imaging probes. Macromol Biosci, 2009, 9: 1227–1236
Striebel HM, Hirschfeld EB, Egerer R, Földes-Papp Z, Tilz GP, Stelzner A. Enhancing sensitivity of human herpes virus diagnosis with DNA microarrays using dendrimers. Exp Mol Pathol, 2004, 77: 89–97
McIntyre JO, Fingleton B, Wells KS, Piston DW, Lynch CC, Gautam S, Matrisian LM. Development of a novel fluorogenic proteolytic beacon for in vivo detection and imaging of tumour-associated matrix metalloproteinase-7 activity. Biochem J, 2004, 377: 617–628
Ternon M, Díaz-Mochón JJ, Belsom A, Bradley M. Dendrimers and combinatorial chemistry-tools for fluorescent enhancement in protease assays. Tetrahedron, 2004, 60: 8721–8728
Iyer AK, Khaled G, Fang J, Maeda H. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov Today, 2006, 11: 812–818
Jevprasesphant R, Penny J, Jalal R, Attwood D, McKeown NB, Emanuele AD. The influence of surface modification on the cytotoxicity of PAMAM dendrimers. Int J Pharm, 2003, 252: 263–266
Boas U, Christensen JB, Heegaard PMH. Dendrimers in Medicine and Biotechnology. London: Roy Soc Chem, 2006
Boas U, Karlsson AJ, Waal BFMD, Meijer EW. Synthesis and properties of new thiourea-functionalized poly(propylene imine) dendrimers and their role as hosts for urea functionalized guests. J Org Chem, 2001, 66: 2136–2145
Boas U, Söntjens SH, Jensen KJ, Christensen JB, Meijer EW. New dendrimer-peptide host-guest complexes: Towards dendrimers as peptide carriers. ChemBioChem, 2002, 3: 433–439
Agrawal P, Umesh Gupta U, Jain NK. Glycoconjugated peptide dendrimers-based nanoparticulate system for the delivery of chloroquine phosphate. Biomaterials, 2007, 28: 3349–3359
Tekade RK, Kumar PV, Jain NK. Dendrimers in oncology: An expanding horizon. Chem Rev, 2009, 109: 49–87
Kolhe P, Khandare J, Pillai O, Kannan S, Lieh-Lai M, Kannan RM. Preparation, cellular transport, and activity of polyamidoamine-based dendritic nanodevices with a high drug payload. Biomaterials, 2006, 27: 660–669
Kurtoglu YE, Navath RS, Wang B, Kannan S, Romero R, Kannan RM. Poly(amidoamine) dendrimer-drug conjugates with disulfide linkages for intracellular drug delivery. Biomaterials, 2009, 30: 2112–2121
Khandare JJ, Jayant S, Singh A, Chandna P, Wang Y, Vorsa N, Minko T. Dendrimer versus linear conjugate: Influence of polymeric architecture on the delivery and anticancer effect of paclitaxel. Bioconjugate Chem, 2006, 17: 1464–1472
Khandare J, Kolhe P, Pillai O, Kannan S, Lieh-Lai M, Kannan RM. Synthesis, cellular transport, and activity of polyamidoamine dendrimer-methylprednisolone conjugates. Bioconjugate Chem, 2005, 16: 330–337
Gurdag S, Khandare J, Stapels S, Matherly LH, Kannan RM. Activity of dendrimer-methotrexate conjugates on methotrexate-sensitive and resistant cell lines. Bioconjugate Chem, 2006, 17: 275–283
Zhuo RX, Du B, Lu ZR. In vitro release of 5-fluorouracil with cyclic core dendritic polymer. J Control Release, 1999, 57: 249–257
Lee CC, Gillies ER, Fox ME, Guillaudeu SJ, Fréchet JM, Dy EE, Szoka FC. A single dose of doxorubicin-functionalized bow-tie dendrimer cures mice bearing C-26 colon carcinomas. Proc Natl Acad Sci USA, 2006, 103: 16649–16654
Okuda T, Kawakami S, Aimoto N, Niidome T, Yamashita F, Hashida M. PEGylated lysine dendrimers for tumor-selective targeting after intravenous injection in tumor-bearing mice. J Control Release, 2006, 116: 330–336
Boyd JB, Kaminskas LM, Karellas P, Krippner G, Lessene R, Porter CJH. Cationic poly-l-lysine dendrimers: pharmacokinetics, biodistribution, and evidence for metabolism and bioresorption after intravenous administration to rats. Mol Pharm, 2006, 3: 614–627
Kaminskas LM, Boyd BJ, Karellas P, Kelly GY, Lessene R, Kelly B, Porter CJH. The impact of molecular weight and PEG chain length on the systemic pharmacokinetics of pegylated poly l-lysine dendrimers. Mol Pharm, 2008, 5: 449–463
Kaminskas LM, Kelly BD, Mchleod VM, Boyd BJ, Krippner GY, Williams ED, Porter CJH. Pharmacokinetics and tumor disposition of PEGylated, methotrexate conjugated poly-l-lysine dendrimers. Mol Pharm, 2009, 6: 1190–1204
Fox ME, Guillaudeu S, Fréchet JMJ, Jerger K, Macaraeg N, Szoka FC. Synthesis and in vivo antitumor efficacy of PEGylated poly (l-lysine) dendrimer-camptothecin conjugates. Mol Pharm, 2009, 6: 1562–1572
Shukla S, Wu G, Chatterjee M, Yang WL, Sekido M, Diop LA, Müller R, Sudimack JJ, Lee RJ, Barth RF, Tjarks W. Synthesis and biological evaluation of folate receptor-targeted boronated pamam dendrimers as potential agents for neutron capture therapy. Bioconjugate Chem, 2003, 14: 158–167
Patri AK, Myc A, Beals J, Thomas TP, Bander NH, Baker JR. Synthesis and in vitro testing of J591 antibody—Dendrimer conjugates for targeted prostate cancer therapy. Bioconjugate Chem, 2004, 15: 1174–1181
Lesniak WG, Kariapper MST, Nair BM, Tan W, Huston A, Balogh LP, Khan MK. Synthesis and characterization of PAMAM dendrimer-based multifunctional nanodevices for targeting αvβ3 integrins. Bioconjugate Chem, 2007, 18: 1148–1154
Chandrasekar D, Sistla R, Ahmad FJ, Khar RK, Diwan PV. The development of folate-PAMAM dendrimer conjugates for targeted delivery of anti-arthritic drugs and their pharmacokinetics and biodistribution in arthritic rats. Biomaterials, 2007, 28: 504–512
Chandrasekar D, Sistla R, Ahmad FJ, Khar RK, Diwan PV. Folate coupled poly(ethyleneglycol) conjugates of anionic poly(amidoamine) dendrimer for inflammatory tissue specific drug delivery. J Biomed Mater Res Part A, 2007, 82A: 92–103
Tam JP. Synthetic peptide vaccine design: Synthesis and properties of a high-density multiple antigenic peptide system. Proc Natl Acad Sci USA, 1988, 85: 5409–5413
Tam JP, Lu YA. Vaccine engineering: enhancement of immunogenicityof synthetic peptide vaccines related to hepatitis in chemically defined models consisting of T- and B-cell epitopes. Proc Natl Acad Sci USA, 1989, 86: 9084–9088
Tam JP, Clavijo P, Lu YA, Nussenzweig V, Nussenzweig R, Zavala F. Incorporation of T and B epitopes of the circumsporozoite protein in a chemically defined synthetic vaccine against malaria. J Exp Med, 1990, 171: 299–306
Oliveira ED, Villén J, Giralt E, Andreu D. Synthetic Approaches to multivalent lipopeptide dendrimers containing cyclic disulfide epitopes of foot-and-mouth disease virus. Bioconjugate Chem, 2003, 14: 144–152
Baigude H, Katsuraya K, Okuyama K, Uryu T. Synthesis of structurally-controlled AIDS vaccine model with glyco-peptide dendrimer scaffolds. Macromol Chem Phys, 2004, 205: 684–691
Gong Y, Matthews B, Cheung D, Tam T, Gadawski I, Leung D, Holan G, Raff J, Sacks S. Evidence of dual sites of action of dendrimers: SPL-2999 inhibits both virus entry and late stages of herpes simplex virus. Antiv Res, 2002, 55: 319–329
Jiang YH, Emau P, Cairns JS, Flanary L, Morton WR, McCarthy TD. SPL7013 gel as a topical microbicide for prevention of vaginal transmission of SHIV89.6P in macaques. AIDS Res Hum Retrov, 2005, 21: 207–213
Tam JP, Lu YA, Yang JL. Antimicrobial dendrimeric peptides. Eur J Biochem, 2002, 269: 923–932
Nagahori N, Lee RT, Nishimura SI, Pagé D, Roy R, Lee YC. Inhibition of adhesion of type 1 fimbriated Escherichia coli to highly mannosylated ligands. ChemBioChem, 2002, 3: 836–844
Kasai S, Nagasawa H, Shimamura M, Uto Y, Hori H. Design and synthesis of antiangiogenic heparin-binding arginine dendrimer mimicking the surface of endostatin. Bioorg Med Chem Lett, 2002, 12: 951–954
Mulligan RC. The basic science of gene therapy. Science, 1993, 260: 926–932
Gu JR, Cao XT. Gene Therapy (in Chinese). Beijing: Sci Press, 2001
Morille M, Passirani C, Vonarbourg A, Clavreul A, Benoit JP. Progress in developing cationic vectors for non-viral systemic gene therapy against cancer. Biomaterials, 2008, 29: 3477–3496
Wu GY, Wu CH. Receptor-mediated in vitro gene transformation by a soluble DNA carrier system. J Biol Chem, 1987, 262: 4429–4432
Mousazadeh M, Palizban A, Salehi R, Salehi M. Gene delivery to brain cells with apoprotein E derived peptide conjugated to polylysine (apoEdp-PLL). J Drug Targ, 2007, 15: 226–230
Dekie L, Toncheva V, Dubruel P, Schacht EH, Barrett L, Seymour LW. Poly-l-glutamic acid derivatives as vectors for gene therapy. J Control Release, 2000, 65: 187–202
Fischer D, Bieber T, Li YX, Elsässer HP, Kissel T. A novel non-viral vector for DNA delivery based on low molecular weight, branched polyethylenimine: Effect of molecular weight on transfection efficiency and cytotoxicity. Pharm Res, 1999, 16: 1273–1279
Ogris M, Brunner S, Schuller S, Kircheis R, Wagner E. PEGylated DNA/transferrin-PEI complexes: Reduced interaction with blood components, extended circulation in blood and potential for systemic gene delivery. Gene Ther, 1999, 6: 595–605
Breunig M, Lungwitz U, Liebl R, Fontanari C, Klar J, Kurtz A, Blunk T, Goepferich A. Gene delivery with tow molecular weight linear polyethytenimines. J Gene Med, 2005, 7: 1287–1298
Boussif O, Lezoualch F, Zanta MA, Mergny MD, Scherman D, Demeneix B, Behr JP. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: Polyethylenimine. Proc Natl Acad Sci USA, 1995, 92: 7297–7301
Gosselin MA, Guo WJ, Lee RJ. Efficient gene transfer using reversibly cross-linked low molecular weight polyethylenimine. Bioconjugate Chem, 2001, 12: 989–994
Jeon O, Yang HS, Lee TJ, Kim BS. Heparin-conjugated polyethyleneimine for gene delivery. J Control Release, 2008, 132: 236–242
Swami A, Aggarwal A, Pathak A, Patnaik S, Kumar P, Singh Y, Gupta KC. Imidazolyl-PEI modified nanoparticles for enhanced gene delivery. Int J Pharm, 2007, 335: 180–192
Ahn HH, Lee JH, Kim KS, Lee JY, Kim MS, Khang G, Lee IW, Lee HB. Polyethyleneimine-mediated gene delivery into human adipose derived stem cells. Biomaterials, 2008, 29: 2415–2422
Zinselmeyer BH, Mackay SP, Schatzlein AG, Uchegbu IF. The lower-generation polypropylenimine dendrimers are effective genetransfer agents. Pharm Res, 2002, 19: 960–967
Russ V, Günther M, Halama A, Ogris M, Wagner E. Oligoethylenimine-grafted polypropylenimine dendrimers as degradable and biocompatible synthetic vectors for gene delivery. J Control Release, 2008, 132: 131–140
Wang J, Mao HQ, Leong KW. A novel biodegradable gene carrier based on polyphosphoester. J Am Chem Soc, 2001, 123: 9480–9481
Kabanov AV, Astafieva IV, Maksimova IV, Lukanidin EM, Georgiev GP, Kabanov VA. Efficient transformation of mammalian cells using DNA interpolyelectrolyte complexes with carbon chain polycations. Bioconjugate Chem, 1993, 4: 448–454
Borchard G. Chitosans for gene delivery. Adv Drug Deliver Rev, 2001, 52: 145–150
Strand SP, Issa MM, Christensen BE, Vårum KM, Artursson P. Tailoring of chitosans for gene delivery: Novel self-branched glycosylated chitosan oligomers with improved functional properties. Biomacromolecules, 2008, 9: 3268–3276
Leong KW, Mao HQ, Le Truong VL, Roy K, Walsh SM, August JT. DNA-polycation nanospheres as non-viral gene delivery vehicles. J Control Release, 1998, 53: 183–193
Wang ZY, Zhao Y, Ren L, Jin LH, Sun LP, Yin P, Zhang YF, Zhang QQ. Novel gelatin-siloxane nanoparticles decorated by Tat peptide as vectors for gene therapy. Nanotechnology, 2008, 19: 445103
Haensler J, Szoka FC. Polyamidoamine cascade polymers mediate efficient transfection of cells in culture, Bioconjugate Chem, 1993, 4: 372–379
Bielinska A, Kukowska-Latallo JF, Johnson J, Tomalia DA, Baker JR. Regulation of in vitro gene expression using antisense oligonucleotides or antisense expression plasmids transfected using starburst PAMAM dendrimers. Nucleic Acids Res, 1996, 24: 2176–2182
Tang MX, Szoka FC. The influence of polymer structure on the interactions of cationic polymers with DNA and morphology of the resulting complexes. Gene Ther, 1997, 4: 823–832
Chen W, Turro NJ, Tomalia DA. Using ethidium bromide to probe the interactions between DNA and dendrimers. Langmuir, 2000, 16: 15–19
Stiriba SE, Frey H, Haag R. Dendritic polymers in biomedical applications: From potential to clinical use in diagnostics and therapy. Angew Chem Int Ed, 2002, 41: 1329–1334
Yamagata M, Kawano T, Shiba K, Mori T, Katayamaa T, Niidome T. Structural advantage of dendritic poly(l-lysine) for gene delivery into cells. Bioorg Med Chem, 2007, 15: 526–532
Coles DJ, Yang S, Esposito A, Mitchell D, Minchin RF, Toth I. The synthesis and characterisation of a novel dendritic system for gene delivery. Tetrahedron, 2007, 63: 12207–12214
Okuda T, Sugiyama A, Niidome T, Aoyagi H. Characters of dendritic poly(l-lysine) analogues with the terminal lysines replaced with arginines and histidines as gene carriers in vitro. Biomaterials, 2004, 25: 537–544
Kim TI, Baek JU, Bai CZ, Park JS. Arginine-conjugated polypropylenimine dendrimer as a non-toxic and efficient gene delivery carrier. Biomaterials, 2007, 28: 2061–2067
Choi JS, Nam K, Park JY, Kim JB, Lee JK, Park JS. Enhanced transfection efficiency of PAMAM dendrimer by surface modification with l-arginine. J Control Release, 2004, 99: 445–456
Duncan R, Izzo L. Dendrimer biocompatibility and toxicity. Adv Drug Delivery Rev, 2005, 57: 2215–2237
Li Y, Zhu YD, Xia KJ, Sheng RL, Jia L, Hou XD, Xu YH, Cao A. Dendritic poly(l-lysine)-b-poly(l-lactide)-b-dendritic poly(l-lysine) amphiphilic gene delivery vectors: Roles of PLL dendritic generation and enhanced transgene efficacies via termini modification. Biomacromolecules, 2009, 10: 2284–2293
Vögtle F, Gestermann S, Hesse R, Schwierz H, Windisch B. Functional dendrimers. Prog Polym Sci, 2000, 25: 987–1041
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gu, Z., Luo, K., She, W. et al. New-generation biomedical materials: Peptide dendrimers and their application in biomedicine. Sci. China Chem. 53, 458–478 (2010). https://doi.org/10.1007/s11426-010-0107-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-010-0107-y