Abstract
Computer-based quantitative structure-activity relationship (QSAR) model has been becoming a powerful tool in understanding the structural requirements for chemicals to bind the estrogen receptor (ER), designing drugs for human estrogen replacement therapy, and identifying potential estrogenic endocrine disruptors. In this study, a simple yet powerful neural network technique, generalized regression neural network (GRNN) was used to develop a QSAR model based on 131 structurally diverse estrogens (training set). Only nine descriptors calculated solely from the molecular structures of compounds selected by objective and subjective feature selections were used as inputs of the GRNN model. The predictive power of the built model was found to be comparable to that of the more traditional techniques but requiring significantly easy implementation and a shorter computation-time. The obtained result indicates that the proposed GRNN model is robust and satisfactory, and can provide a feasible and practical tool for the rapid screening of the estrogenic activity of organic compounds.
Similar content being viewed by others
References
Cooper R L, Kavlock R J. Endocrine disruptors and reproductive development: A weight-of-evidence overview. J Endocrinol, 1997, 152(2): 159–166
Kavlock R J, Daston G P, DeRosa C, Fenner-Crisp P, Gray L E, Kaattari S, Lucier G, Luster M, Mac M J, Maczka C, Miller R, Moore J, Rolland R, Scott G, Sheehan D M, Sinks T, Tilson H A. Research needs for the risk assessment of health and environmental effects of endocrine disruptors: A report of the U.S. EPA sponsored workshop. Environ Health Persp, 1996, 104(Suppl 4): 715–740
Asikainen A, Kolehmainen M, Ruuskanen J, Tuppurainen K. Structure-based classification of active and inactive estrogenic compounds by decision tree, LVQ and kNN methods. Chemosphere, 2006, 62(4): 658–673
Waller C L, Oprea T I, Chae K, Park H K, Korach K S, Laws S C, Wiese T E, Kelce W R, Gray L E Jr. Ligand-based identification of environmental estrogens. Chem Res Toxicol, 1996, 9(8): 1240–1248
Tong W, Perkins R, Xing L, Welsh W J, Sheehan D M. QSAR models for binding of estrogenic compounds to estrogen receptor alpha and beta subtypes. Endocrinology, 1997, 138(9): 4022–4025
Shi L M, Fang H, Tong W, Wu J, Perkins R, Blair R M, Branham W S, Dial S L, Moland C L, Sheehan D M. QSAR models using a large diverse set of estrogens. J Chem Inf Comput Sci, 2001, 41(1): 186–195
Yu S J, Keenan S M, Tong W, Welsh W J. Influence of the structural diversity of data sets on the statistical quality of 3D-QSAR models: Predicting the estrogenic activity of xenoestrogens. Chem Res Toxicol, 2002, 15(10): 1229–1234
Colemana K P, Toscano W A, Wiese T E. QSAR Models of the in vitro estrogen activity of bisphenol A analogs. QSAR Comb Sci, 2003, 22: 78–88
Asikainen A, Ruuskanen J, Tuppurainen K. Spectroscopic QSAR methods and self-organizing molecular field analysis for relating molecular structure and estrogenic activity. J Chem Inf Comput Sci, 2003, 43(6): 1974–1981
Waller C L. A comparative QSAR study using CoMFA, HQSAR and FRED/SKEYS paradigms for estrogen receptor binding affinities of structurally diverse compounds. J Chem Inf Comput Sci, 2004, 44: 758–765
Lill M A, Vedani A, Dobler M. Raptor: Combining dual-shell representation, Induced-fit simulation, and hydrophobicity scoring in receptor modeling: Application toward the simulation of structurally diverse ligand sets. J Med Chem, 2004, 47(25): 6174–6186
Marini F, Roncaglioni A, Novic M. Variable selection and interpretation in structure-affinity correlation modeling of estrogen receptor binders. J Chem Inf Model, 2005, 45: 1507–1519
Salomon R, Hemmen J. Accelerating back propagation through dynamic self-adaptation. Neual Networks, 1996, 9(4): 589–601
Bolanca T, Cerjan-Stefanovic S, Regelja M, Regelja H, Loncaric S. Development of an inorganic cations retention model in ion chromatography by means of artificial neural networks with different two-phase training algorithms. J Chromatogr A, 2005, 1085: 74–85
Engin M, Demirag S, Engin E, Celbi G, Ersan F, Asena E, Colakoglu Z. The classification of human tremor signals using artificial neural network. Expert Syst Appl, 2007, 33: 754–761
Yao X J, Panaye A, Doucet J P. Comparative study of qsar/qspr correlations using support vector machines, radial basis function neural networks, and multiple linear regression. J Chem Inf Model, 2004, 44: 1257–1266
Liu H, Papa E, Gramatica P. QSAR prediction of estrogen activity for a large set of diverse chemicals under the guidance of OECD principles. Chem Res Toxicol, 2006, 19: 1540–1548
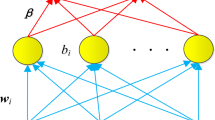
Specht D F A. General Regression Neural Network. IEEE T Neural Network, 1991, 2(6): 568–576
Chtioui Y, Panigrahi S, Francl L. A generealized regression neural network and its application for leaf wetness prediction to forecast plant disease. Chemometr Intell Lab Syst, 1999, 48: 47–58
Tomoko N. Using general regression and probabilistic neural networks to predict human intestinal absorption with topological descriptors derived from two-dimensional chemical structures. J Chem Inf Comput Sci, 2003, 43: 113–119
Philip D M, Peter C J. QSAR/QSPR studies using probabilistic neural networks and generalized regression neural networks. J Chem Inf Comput Sci, 2002, 42: 1460–1470
Johanna K, Bettina W, Gerhard B, Klocker J, Wailzer B, Buchbauer G, Wolschann P. Bayesian neural networks for aroma classification. J Chem Inf Comput Sci, 2002, 42: 1443–1449
Hilmi B C. Application of radial basis function and generalized regression neural networks in non-linear utility function specification for travel mode choice modeling. Math Comput Model, 2006, 44: 640–658
Kohonen T. Self-organized formation of topologically correct feature maps. Biol Cybern, 1982, 43: 59–69
Kohonen T, Hynninen J, Kangas J, Laaksonen J. The Self-Oraganizing Map Program Package. Technical Report A31, Helsinki University of Technology, Laboratory of Computer and Information Science, FIN-02150 Espoo, Finland, 1996. Software available at http://www.cis.hut.fi/research/som_pak
Kuiper G G, Lemmen J G, Carlsson B, Corton J C, Safe S H, Saag P T, Burg B, Gustafsson J A. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology, 1998, 139: 4252–4263
Todeschini R, Consonni V, Mauri A, Pavan M M. Dragon 2.1. Chemometrics and QSAR Research Group, University of Milano-Bicocca, Milan, Italy, 2002
Todeschini R, Consonni V. Handbook of Molecular Descriptors. Germany: Wiley-VCH, 2000
Johhnson S R. Prediction of physicochemical properties and biological activities from molecular structure and the use of computational neural networks for the analysis of sensor apply data. Doctor Dissertation. Pennsylvania: The Pennsylvania State University, 1999
Mcelroy N R. The prediction of physical properties and biological activities of orgnanic compounds from their molecular structures. Doctor Dissertation. Pennsylvania: The Pennsylvania State University, 2003
So S, Karplus M. Evolutionary optimization in quantitative structure-activity relationship: An application of genetic neural networks. J Med Chem, 1996, 39: 1521–1530
Rogers D, Hopfinger A J. Application of genetic function approximation to quantitative structure-activity relationships and quantitative structure-property relationships. J Chem Inf Comput Sci, 1994, 34: 854–866
Boilot P, Hines E L, Gongora M A, Folland R S. Electronic noses inter-comparison, data fusion and sensor selection in discrimination of standard fruit solutions. Sens Actuat B-Chem, 2003, 88: 80–88
Massart D L, Vandeginste B G M, Buydens L M C. Handbook of Chemometrics and Qualimetrics, part A. Netherlands: Elsevier, 1997
Luke B T. Evolutionary programming applied to the development of quantitative structure activity relationships and quantitative structure property relationships. J Chem Inf Comput Sci, 1994, 34: 1279–1287
Fang H, Tong W, Shi L, Blair R, Perkins R, Branham W S, Dial S L, Moland C L, Sheehan D M. Structure activity relationship for a large diverse set of natural, synthetic and environmental chemicals. Chem Res Toxicol, 2001, 14: 280–294
Tong W, Fang H, Hong H, Xie Q, Perkins R, Anson1 J, Sheehan D M. Regulatory application of SAR/QSAR for priority setting of endocrine disruptors: A perspective. Pure Appl Chem, 2003, 75: 2375–2388
Asikainen A, Ruuskanen J, Tuppurainen K. Consensus kNN QSAR: A versatile method for predicting the estrogenic activity of organic compounds in silico. A comparative study with five estrogen receptors and a large, diverse set of ligands. Environ Sci Technol, 2004, 38(24): 6724–6729
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the National Natural Science Foundation of China (Grant Nos. 20507008 and 20737001), the Natural Science Foundation of Jiangsu Province, China (Grant No. BK200418) and the National Basic Research Program of China (973 Program)(Grant No. 2003CB415002)
Rights and permissions
About this article
Cite this article
Ji, L., Wang, X., Luo, S. et al. QSAR study on estrogenic activity of structurally diverse compounds using generalized regression neural network. Sci. China Ser. B-Chem. 51, 677–683 (2008). https://doi.org/10.1007/s11426-008-0070-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-008-0070-z