Abstract
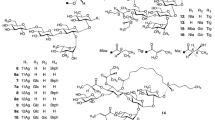
Calystegia hederacea Wall. (Convolvulaceae) is a perennial herbaceous vine that grows widely in India and East Asia. All parts of this plant are used to treat various disorders such as menoxenia and gonorrhea. Four new resin glycosides, calyhedins XI (1)–XIV (4), were isolated from the rhizomes of C. hederacea. A new glycoside, calyhedin XV (5), was isolated from its leaves and stems. Alkaline hydrolysis of 1 and 2 furnished a new glycosidic acid, calyhedic acid G (1a), from 1 and a new acid, calyhedic acid H (2a), from 2 along with 2S-methylbutyric acid and 2R-methyl-3R-hydroxybutyric (2R,3R-nilic) acid. The structures of 1–5, 1a, and 2a were determined using MS and NMR spectral analyses. Compounds 1a and 2a had the same sugar moiety, β-d-glucopyranosyl-(1 → 6)-O-β-d-glucopyranosyl-(1 → 6)-O-β-d-glucopyranosyl-(1 → 3)-[O-β-d-glucopyranosyl-(1 → 3)-O-α-l-rhamnopyranosyl-(1 → 2)]-O-β-d-glucopyranosyl-(1 → 2)-β-d-fucopyranose, while their aglycones were 11S-dihydroxyhexadecanoic acid and 12S-dihydroxyhexadecanoic acid, respectively. These compounds are the first glycosidic acids, with fucose as the monosaccharide component obtained from the resin glycosides of C. hederacea. Compounds 1–5, comprising either 1a or 2a, were heptaglycosides with macrolactone structures, and their sugar moieties were partially acylated with 5 mol of organic acids comprising 2S-methylbutyric, (E)-2-methylbut-2-enoic, and 2R,3R-nilic acids. Compounds 1 and 5 had 22-membered rings, while 2–4 had 28-membered rings. In addition, 1 and 5 exhibited cytotoxic activity against HL-60 human promyelocytic leukemia cells, comparable to that of the positive control cisplatin.
Graphical abstract





Similar content being viewed by others
References
Ono M (2017) Resin glycosides from Convolvulaceae plants. J Nat Med 71:591–604
Useful plants of the world (1989) In: Hotta M, Ogata K, Nitta A, Hosikawa K, Yanagi M, Yamazaki K (eds) Heibonsha Ltd., Tokyo, Japan, p. 196 (in Japanese plant dictionary)
Ono M, Ichihara Y, Saito N, Yamada M, Yuuki K, Nawata M, Tsutsumi S, Yasuda S, Tsuchihashi R, Okawa M, Kinjo J, Miyashita H, Yoshimitsu H, Nohara T (2020) Identification and characterization of organic and glycosidic acids in crude resin glycoside fraction from Calystegia hederacea. J Nat Med 74:200–211
Ono M, Saito N, Minamishima H, Yasuda S, Tsuchihashi R, Okawa M, Kinjo J, Miyashita H, Yoshimitsu H, Nohara T (2022) Two new glycosidic acids, calyhedic acids E and F, in crude resin glycoside fraction from Calystegia hederacea. Nat Prod Res 36:46–53
Ono M, Yuhara N, Shimohara T, Matsubara S, Yasuda S, Tsuchihashi R, Okawa M, Kinjo J, Zhou JR, Yoshimitsu H, Nohara T (2021) Calyhedins I-VI: Resin glycosides from the rhizomes of Calystegia hederacea. Phytochemistry. https://doi.org/10.1016/j.phytochem.2021.112888
Ono M, Shimohara T, Yuhara N, Matsubara S, Yasuda S, Tsuchihashi R, Okawa M, Kinjo J, Yoshimitsu H, Nohara T (2021) Four new resin glycosides, calyhedins VII–X, from the rhizomes of Calystegia hederacea. Nat Prod Res. https://doi.org/10.1080/14786419.2021.2005593
Tanaka T, Nakashima T, Ueda T, Tomii K, Kouno I (2007) Facile discrimination of aldose enantiomers by reversed-pase HPLC. Chem Pharm Bull 55:899–901
Ono M, Azuchi M, Ichio M, Jiyoubi Y, Tsutsumi S, Yasuda S, Tsuchihasi R, Okawa M, Kinjo J, Yoshimitsu H, Nohara T (2019) Seven new resin glycosides from the seeds of Quamoclit × multifida. J Nat Med 73:11–22
Ono M, Taketomi S, Nishikawa H, Yasuda S, Tsuchihashi R, Okawa M, Kinjo J, Miyashita H, Yoshimitsu H, Nohara T (2022) Two new resin glycosides, muricatins XII and XIII, from the seeds of Ipomoea muricata. Nat Prod Res, https://doi.org/10.1080/14786419.2022.2125970
Seo S, Tomita Y, Tori K, Yoshimura Y (1978) Determination of the absolute configuration of a secondary hydroxy group in a chiral secondary alcohol using glycosidation shifts in carbon-13 nuclear magnetic resonance spectroscopy. J Am Chem Soc 100:3331–3339
Ono M, Honda F, Karahashi A, Kawasaki T, Miyahara K (1997) Resin glycosides. XXV. Multifidins I and II, new jalapins, from the seed of Quamoclit × multifida. Chem Pharm Bull 45:1955–1960
Kasai R, Suzuo M, Asakawa J, Tanaka O (1977) Carbon-13 chemical shifts of isoprenoid-β-D-glucopyranosides and - β -D-mannopyranosides. Stereochemical influences of aglycone alcohols. Tetrahedron Lett 2:175–178
Tori K, Seo S, Yoshimura Y, Arita H, Tomita Y (1977) Glycosidation shifts in carbon-13 NMR spectroscopy: carbon-13 signal shifts from aglycone and glucose to glucoside. Tetrhedron Lett 2:179–182
Noda N, Ono M, Miyahara K, Kawasaki T (1987) Resin glycosides. I. Isolation and structure elucidation of orizabin-I, II, III and IV, genuine resin glycosides from the root of Ipomoea orizabensis. Tetrahedron 43:3889–3902
Acknowledgements
We express our appreciation to Mr. H. Harazono of Fukuoka University for the measurement of the FAB-MS and ESI-TOF-MS. This research was partially supported by a Grant-in-Aid for Scientific Research (C) (JSPS KAKENHI Grant Numbers JP16K08306 and JP20K07117) and by the Research and Study Project of Tokai University General Research Organization (Kanagawa, Japan).
Funding
This research was partially supported by a Grant-in-Aid for Scientific Research (C) (JSPS KAKENHI Grant Numbers JP16K08306 and JP20K07117).
Author information
Authors and Affiliations
Contributions
Dr. Ono supervised the research, wrote the manuscript, and analyzed the data. Mr. Yamano, Mr. Shimohara, and Mr. Yuhara purified the compounds. Ms. Misuda, Mr. Nishikawa, and Dr. Yasuda were responsible for the antitumor activity. Drs. Miyashita, Yoshimitsu, Tsuchihasi, Okawa, and Kinjo performed instrumental analysis and data analysis.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ono, M., Yamano, Y., Shimohara, T. et al. Five new resin glycosides, calyhedins XI–XV, from Calystegia hederacea. J Nat Med 77, 774–791 (2023). https://doi.org/10.1007/s11418-023-01720-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-023-01720-y