Abstract
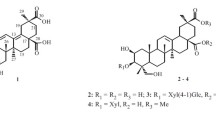
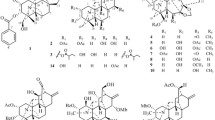
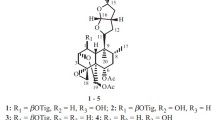
Twenty new neo-clerodane type diterpenoids, scutefolides G1-S (1–20), were isolated from the 70 % aqueous acetone extract of the aerial parts of Scutellaria coleifolia. Their structures were elucidated by extensive spectroscopic analyses. The absolute configurations of 1, 2, 7, 8, 14 and 15 were determined by means of the CD exciton chirality method. Compounds 1, 2, 5, 7, 8, 12, 14, 15, 18 and 19 were evaluated for their cytotoxic activities against four human cancer cell lines, and anti-bacterial activities against methicillin-resistant Staphylococcus aureus.




Similar content being viewed by others
References
Shang X, He X, He X, Li M, Zhang R, Fan P, Zhang Q, Jia Z (2010) The genus Scutellaria an ethnopharmacological and phytochemical review. J Ethnopahrmacol 128:279–313
Ministry of Health (2011) Labour and Welfare of Japan (in Japanese). The Japanese Pharmacopoeia, 16th edn. Tokyo
Pharmacopoeia Commission Ministry of Public Health (2000) Pharmacopoeia of the People’s Republic in China. Part 1 (in Chinese). Beijing
Wang TS, Wang SQ, Xiao DL (2012) A review of phytochemistry and antitumor activity of a valuable medicinal species: Scutellaria barbata. J Med Plants Res 6:4259–4275
Kurimoto S, Pu JX, Sun HD, Takaishi Y, Kashiwada Y (2015) Coleifolides A and B, two new sesterterpenoids from the aerial parts of Scutellaria cleifolia Levl. Chem Biodivers 12:1200–1207
Kurimoto S, Pu JX, Sun HD, Takaishi Y, Kashiwada Y (2015) Acylated neo-clerodanes and 19-nor-neo-clerodanes from the aerial parts of Scutellaria coleifolia (Lamiaceae). Phytochemistry 116:298–304
Bozov PI, Malakov PY, Papanov GY, de la Torre MC, Rodríguez B, Perales A (1993) Scutalpin A, a neo-clerodane diterpene from Scutellaria alpina. Phytochemistry 34:453–456
Lin YL, Kuo YL (1989) Four new neoclerodane-type diterpenoids, scutellones B, G, H, and I, from aerial parts of Scutellaria rivularis. Chem Pharm Bull 37:582–585
de la Torre MC, Rodríguez B, Bruno M, Piozzi F, Savona G, Vassallo N, Servettaz O (1995) Neo-Clerodane diterpenoids from Scutellaria alpina. Phytochemistry 38:181–187
Malakov PY, Papanov GY (1996) Neo-clerodane diterpenoids from Scutellaria orientalis subsp. pinnatifida. Phytochemistry 43:173–178
Kizu H, Imoto Y, Tomimori T, Kikuchi T, Kadota S, Tsubono K (1997) Studies on the constituents of Scutellaria species. XVIII. Structures of neoclerodane-type diterpenoids from the whole herb of Scutellaria rivularis Wall. Chem Pharm Bull 45:152–160
Muñoz DM, de la Torre MC, Rodríguez B, Simmonds MSJ, Blaney WM (1997) Neo-clerodane insect antifeedants from Scutellaria alpina subsp. javalambrensis. Phytochemistry 44:593–597
Esquivel B, Domínguez RM, Toscano RA (2001) Neoclerodane diterpenoids from Scutellaria caerulea. J Nat Prod 64:778–782
Nguyen VH, Pham VC, Nguyen TTH, Tran VH, Doan TMH (2009) Novel antioxidant neo-clerodane diterpenoids from Scutellaria barbata. Eur J Org Chem 2009:5810–5815
Lee H, Kim Y, Choi I, Min BS, Shim SH (2010) Two novel neo-clerodane diterpenoids from Scutellaria barbata. Bioorg Med Chem Lett 20:288–290
Zhu F, Di YT, Liu LL, Zhang Q, Fang Q, Yang TQ, Hao XJ, He HP (2010) Cytotoxic neoclerodane diterpenoids from Scutellaria barbata. J Nat Prod 73:233–236
Nie XP, Qu GW, Yue XD, Li GS, Dai SJ (2010) Scutelinquanines A–C, three new cytotoxic neo-clerodane diterpenoid from Scutellaria barbata. Phytochem Lett 3:190–193
Wang F, Ren FC, Li YJ, Liu JK (2010) Scutebarbatines W–Z, new neo-clerodane diterpenoids from Scutellaria barbata and structure revision of a series of 13-spiro neo-clerodanes. Chem Pharm Bull 58:1267–1270
Raccuglia RA, Bellone G, Loziene K, Piozzi F, Rosselli S, Maggio A, Bruno M, Simmonds MSJ (2010) Hastifolins A–G, antifeedant neo-clerodane diterpenoids from Scutellaria hastifolia. Phytochemistry 71:2087–2091
Lee H, Shim SH (2011) neo-Clerodane diterpenoids from the aerial parts of Scutellaria barbata. Helv Chim Acta 94:643–649
Dai SJ, Peng WB, Zhang DW, Shen L, Wang WY, Ren Y (2009) J Nat Prod 72:1793–1797
Dai SJ, Tao JY, Liu K, Jiang YT, Shen L (2006) neo-Clerodane diterpenoids from Scutellaria barbata with cytotoxic activities. Phytochemistry 67:1326–1330
Miyaichi Y, Kizu H, Yamaguchi Y, Tomimori T (1994) Studies on the constituents of Scutellaria spices. XV. On the diterpenoid constituents of the leaves of Scutellaria alpina L. Yakugaku Zasshi 114:264–271
Hussein AA, de la Torre MC, Jimeno ML, Rodríguez B, Bruno M, Piozzi F, Servettaz O (1996) A neo-clerodane diterpenoids from Scutellaria baicalensis. Phytochemistry 43:835–837
de la Torre MC, Rodríguez B, Bruno M, Vassallo N, Bondì ML, Piozzi F, Servettaz O (1997) Neoclerodane diterpenoids from Scutellaria polyodon. J Nat Prod 60:1229–1235
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Shibata H, Kondo K, Katsuyama R, Kawazoe K, Sato Y, Murakami K, Takaishi Y, Arakaki N, Higuti T (2005) Alkyl gallates, intensifiers of β-lactam susceptibility in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 49:549–555
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Kurimoto, Si., Pu, JX., Sun, HD. et al. Acylated neo-clerodane type diterpenoids from the aerial parts of Scutellaria coleifolia Levl. (Lamiaceae). J Nat Med 70, 241–252 (2016). https://doi.org/10.1007/s11418-016-0967-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-016-0967-3