Abstract
Purpose
Biochars have considerable potential to improve soil organic carbon (SOC) sequestration and consequently regulate greenhouse gas emissions. However, the important roles of microbial mediation in the biochar-induced SOC accumulation over the plant growth season have not been adequately explored. Here, we illustrated the interactive effects of biochar amendments and plant growth stages on the microbial community and SOC mineralisation.
Materials and methods
A 3-year experiment was performed in a tobacco (Nicotiana tabacum L.) field with five treatments, including no fertilisation, conventional fertilisation, and conventional fertilisation with three rates of biochar amendments.
Results and discussion
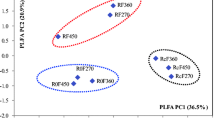
Biochar amendments significantly improved soil moisture capacity (SMC) but decreased nitrogen (N) availability. The bacterial and fungal biomasses were enriched under biochar amendments and at the rosette and vigorous stages of the crop growing season. Biochar amendments and plant growth stages substantially affected the microbial community structure, as determined by the ratios of bacteria to fungi (B/F) and Gram-positive bacteria to Gram-negative bacteria (GP/GN). Random forest modelling revealed that SMC and N availability were the important predictors of microbial community and SOC mineralisation. Structural equation modelling indicated that microbial biomass and community structure (the rations of B/F and GP/GN) were positively associated with SMC but negatively correlated with N availability. Microbial community structure was more influential than microbial biomass in reducing microbial carbon metabolism of carbohydrates (cellobiose, glucose, and xylose) and SOC mineralisation.
Conclusions
Our study provided insights into the functional role of the microbial community in the biochar-induced negative priming effect on SOC mineralisation during the plant growth stages.






Similar content being viewed by others
References
Anderson MJ, Willis TJ (2003) Canonical analysis of principal coordinates: a useful method of constrained ordination for ecology. Ecology 84:511–525
Andrews M, Raven JA, Lea PJ (2013) Do plants need nitrate? The mechanisms by which nitrogen form affects plants. Ann Appl Biol 163:174–199
Archer E (2016) Rfpermute: estimate permutation p-values for random forest importance metrics. R package version 2.0.1.
Atkinson CJ, Fitzgerald JD, Hipps NA (2010) Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: a review. Plant Soil 337:1–18
Breiman L (2001) Random forests. Mach Learn 45:5–32
Bruun EW, Ambus P, Egsgaard H, Hauggaard-Nielsen H (2012) Effects of slow and fast pyrolysis biochar on soil C and N turnover dynamics. Soil Biol Biochem 46:73–79
Chen L, Jiang Y, Liang C, Luo Y, Xu Q, Han C, Zhao Q, Sun B (2019) Competitive interaction with keystone taxa induced negative priming under biochar amendments. Microbiome 7:77
Clarke KR, Gorley RN (2006) PRIMER v6: User manual/tutorial (Plymouth Routines in Multivariate Ecological Research). Plymouth Marine Laboratory, United Kingdom
Farrell M, Kuhn TK, Macdonald LM, Maddern TM, Murphy DV, Hall PA, Singh BP, Baumann K, Krull ES, Baldock JA (2013) Microbial utilization of biochar-derived carbon. Sci Total Environ 465:288–297
Fortmann-Roe S (2013) Accurate, adaptable, and accessible error metrics for predictive. R Package Version 0.9.2.
Frostegård A, Bååth E (1996) The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biol Fertil Soils 22:59–65
Frostegård A, Bååth E, Tunlid A (1993) Shifts in the structure of soil microbial communities in limed forests as revealed by phospholipid fatty acid analysis. Soil Biol Biochem 25:723–730
Grace JB (2006) Structural equation modeling and natural systems. Cambridge University Press.
Grogan DW, Cronan JE (1997) Cyclopropane ring formation in membrane lipids of bacteria. Microbiol Mol Biol Rev 61:429–441
Gul S, Whalen JK (2016) Biochemical cycling of nitrogen and phosphorus in biochar-amended soils. Soil Biol Biochem 103:1–15
Gul S, Whalen JK, Thomas BW, Sachdeva V, Deng H (2015) Physico-chemical properties and microbial responses in biochar-amended soils: mechanisms and future directions. Agric Ecosyst Environ 206:46–59
Hendershot WH, Lalande H, Duquette M (2007) Ion exchange and exchangeable cations. In: Carter MR, Gregorich EG (eds) Soil sampling and methods of analysis (2nd ed) part III-soil chemical analyses. CRC Press, Boca Raton, pp 203–206
Herath H, Camps-Arbestain M, Hedley M (2013) Effect of biochar on soil physical properties in two contrasting soils: an Alfisol and an Andisol. Geoderma 209:188–197
Hooper D, Coughlan J, Mullen M (2008) Structural equation modelling: guidelines for determining model fit. Electron J Bus Res Methods 6:53–60
Jeffery S, Verheijen FGA, Velde MVD, Bastos AC (2011) A quantitative review of the effects of biochar application to soils on crop productivity using meta-analysis. Agric Ecosyst Environ 144:175–187
Jury WA, Gardner WR, Gardner WH (1991) Water characteristic function. In: Jury WA, Gardner WR, Gardner WH (eds) Soil physics (5th ed) part 2−water retention in soil. John Wiley & Sons, Inc, New York, pp 61–67
Kanehiro Y, Sherman GD (1965) Fusion with sodium carbonate for total elemental analysis. In: Black CA (ed) Methods of soil analysis, part 2–agronomy 9. America Society of Agronomy, Inc, Madison, pp 952–958
Karhu K, Mattila T, Bergström I, Regina K (2011) Biochar addition to agricultural soil increased CH4 uptake and water holding capacity—results from a short-term pilot field study. Agric Ecosyst Environ 140:309–313
Keith A, Singh B, Dijkstra FA (2015) Biochar reduces the rhizosphere priming effect on soil organic carbon. Soil Biol Biochem 88:372–379
Kieft TL, Wilch E, Oconnor K, Ringelberg DB, White DC (1997) Survival and phospholipid fatty acid profiles of surface and subsurface bacteria in natural sediment microcosms. Appl Environ Microbiol 63:1531–1542
Lee SH, Kim CG, Kang H (2011) Temporal dynamics of bacterial and fungal communities in a genetically modified (GM) rice ecosystem. Microb Ecol 61:646–659
Lehmann J, Rillig MC, Thies J, Masiello CA, Hockaday WC, Crowley D (2011) Biochar effects on soil biota–A review. Soil Biol Biochem 43:1812–1836
Li XZ, Rui JP, Mao YJ, Yannarell A, Mackie R (2014) Dynamics of the bacterial community structure in the rhizosphere of a maize cultivar. Soil Biol Biochem 68:392–401
Liaw A, Wiener M (2002) Classification and regression by randomforest. R News 2:18–22
Liu QS, Liu Y, Show KY, Tay JH (2009) Toxicity effect of phenol on aerobic granules. Environ Technol 30:69–74
Lv F, Luo CH, Shao LM, He PJ (2016) Biochar alleviates combined stress of ammonium and acids by firstly enriching Methanosaeta and then Methanosarcina. Water Res 90:34–43
Maestrini B, Nannipieri P, Abiven S (2015) A meta-analysis on pyrogenic organic matter induced priming effect. GCB Bioenergy 7:577–590
Mentzer JL, Goodman RM, Balsar TC (2006) Microbial response over time to hydrologic and fertilizations treatments in a simulated wet prairie. Plant Soil 284:85–100
Nguyen TTN, Xu CY, Tahmasbian I, Che RX, Xu ZH, Zhou XH, Wallace HM, Bai SH (2017) Effects of biochar on soil available inorganic nitrogen: A review and meta-analysis. Geoderma 288:79–96
Nguyen TTN, Wallace HM, Xu CY, Zwieten L, Weng ZH, Xu ZH, Che RX, Tahmasbian I, Hu HW, Shahla BH (2018) The effects of short term, long term and reapplication of biochar on soil bacteria. Sci Total Environ 636:142–151
Novak JM, Lima I, Xing B, Gaskin JW, Steiner C, Das KC, Ahmedna M, Rehrah D, Watts DW, Busscher WJ, Schomberg H (2009) Characterization of designer biochar produced at different temperatures and their effects on a loamy sand. Annu Environ Sci 3:195–206
O’Halloran IP, Cade-Menun BJ (2007) Total and organic phosphorus. In: Carter MR, Gregorich EG (eds) Soil sampling and methods of analysis (2nd ed) part III−soil chemical analyses. CRC Press, Boca Raton, pp 267–271
Olsen SR, Cole C, Watanabe FS, Dean L (1954) Estimation of available phosphorus in soils by extraction with sodium bicarbonate. Circular (No. 939). USDA Press, Washington DC, pp 1–19
Quilliam RS, Glanville HC, Wade SC, Jones DL (2013) Life in the ‘charosphere’ −does biochar in agricultural soil provide a significant habitat for microorganisms? Soil Biol Biochem 65:287–293
Sparks DL, Page AL, Helmke PA, Loeppert RH, Mulvaney RL (1965) Nitrogen−inorganic forms. In: Black CA (ed) Methods of soil analysis part 2−agronomy 9. America Society of Agronomy, Inc, Madison, pp 1149–1224
Steiner C (2008) Biochar carbon sequestration. Biorefining and Carbon Cycling Program. University of Georgia, Athens, GA
Trivedi P, Anderson IC, Singh BK (2013) Microbial modulators of soil carbon storage: integrating genomic and metabolic knowledge for global prediction. Trends Microbiol 21:641–651
Ursino N, Contarini S (2006) Stability of banded vegetation patterns under seasonal rainfall and limited soil moisture storage capacity. Adv Water Resour 29:1556–1564
Wang L, Macko SA (2011) Constrained preferences in nitrogen uptake across plant species and environments. Plant Cell Environ 34:525–534
Whitman T, Pepe-Ranney C, Enders A, Koechli C, Campbell A, Buckley DH, Lehmann J (2016) Dynamics of microbial community composition and soil organic carbon mineralization in soil following addition of pyrogenic and fresh organic matter. ISME J 10:2918–2930
Woolf D, Amonette JE, Street FA, Lehmann J, Joseph S (2010) Sustainable biochar to mitigate global climate change. Nat Commun 1:1–9
Zelles L (1999) Fatty acid patterns of phospholipids and lipopolysaccharides in the characterisation of microbial communities in soil: a review. Biol Fertil Soils 29:111–129
Zheng H, Wang ZY, Deng X, Herbert S, Xing BS (2013) Impacts of adding biochar on nitrogen retention and bioavailability in agricultural soil. Geoderma 206:32–39
Zheng H, Wang X, Luo XX, Wang ZY, Xing BS (2018) Biochar-induced negative carbon mineralization priming effects in a coastal wetland soil: Roles of soil aggregation and microbial modulation. Sci Total Environ 610:951–960
Zimmerman AR, Gao B, Ahn MY (2011) Positive and negative carbon mineralization priming effects among a variety of biochar-amended soils. Soil Biol Biochem 43:1169–1179
Funding
This research was financially supported by the National Key Research and Development Project (2016YFD0200309), National Science Fund for Excellent Young Scholars of China (41922048), National Natural Science Foundation of China (41530856, 41771297), Outstanding Youth Scholar Program of Jiangsu Province (BK20180049), Natural Science Foundation of Jiangsu Province (BK20171520), and Youth Innovation Promotion Association of CAS (2017361).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Weijin Wang
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1727 kb)
Rights and permissions
About this article
Cite this article
Wang, H., Zhang, W., Chen, L. et al. Biochar induced negative priming effect on soil organic carbon mineralisation by changing the microbial community structure across plant growth stages. J Soils Sediments 20, 3340–3350 (2020). https://doi.org/10.1007/s11368-020-02662-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-020-02662-8