Abstract
Purpose
Purple paddy soils cover about 66% of the paddy soils in Sichuan Basin. Complex interactions made the effects of potassium fertilizer application on NH4+ adsorption more complicated and the behavior of NH4+ and K+ interaction in purple paddy soils has rarely been evaluated. We hypothesize that correct management of these soils can enhance the efficiency of ammonium and potassium fertilizers. In order to determine correct management methods, it is important to understand the interaction of main nutrient cations NH4+ and K+ and maturation hydroponics in purple paddy soils with different paddy cultivation times.
Materials and methods
A purple paddy soil chronosequence with cultivation history from 0 to 300 years was studied to understand the respective effects of SOM and mineralogy on NH4+ and K+ sorption behavior by adsorption experiments using samples both before and after H2O2 oxidation.
Results and discussion
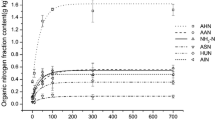
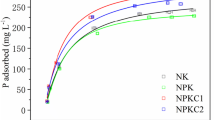
The study shows that maturation hydroponics influenced NH4+ and K+ adsorption mainly through changing clay mineralogy and enhancing SOM accumulation. SOM and smectite play positive effects for NH4+ adsorption. The K+ adsorption, however, is mainly affected by the smectite changes. The adsorption percentages are obviously higher at 25–50 mg L−1 for each NH4+ solution and higher at 50–100 mg L−1 for each K+ solution. When ammonium and potassium were applied together, NH4+ and K+ adsorption are mutually weakened. In addition, the percentage of K+ adsorption increased from 49.7–57.7% for the 100 mg L−1 K+ solution to 50.9–58.5% for the 200 mg L−1 K+ solution at 200 mg L−1 of added NH4+.
Conclusions
SOM enrichments could be a positive way to improve the ammonium fertilizer utilization efficiency in purple paddy soils. Potassium may be more vulnerable to loss at low K+ concentrations given that ammonium and potassium fertilizers were applied together and it should be separately applied to enhance the utilization efficiency of applied fertilizer.




Similar content being viewed by others
References
Balci S, Dincel Y (2002) Ammonium ion adsorption with sepiolite: use of transient uptake method. Chem Eng Process 41:79–85
Brown G, Brindley GW (1980) X-ray diffraction procedures for clay mineral identification. In: Crystal structures of clay minerals and their x-ray identification, Mineralogical Society Monograph No. 5. Mineralogical Society, London, pp 305-359
Buragohain P, Sreedeep S, Lin P, Ni J, Garg A (2019) Influence of soil variability on single and competitive interaction of ammonium and potassium: experimental study on seven different soils. J Soils Sediments 19:186–197
Cai GX, Peng GH, Wang XZ, Zhu JW, Zhu ZL (1992) Ammonia volatilization from urea applied to acid paddy soil in Southeast China and its control. Pedosphere 2:345–354
Chappell MA, Evangelou VP (2000) Influence of added K+ on inducing ammonium fixation and inhibiting nitrification. Soil Sci 165:420–426
Chen JS, Mackenzie AF (1992) Fixed ammonium and potassium as affected by added nitrogen and potassium in three Quebec soils. Commun Soil Sci Plan 23:1145–1159
Clément JC, Shrestha J, Ehrenfeld JG, Jaffe PR (2005) Ammonium oxidation coupled to dissimulatory reduction of iron under anaerobic conditions in wetland soils. Soil Biol Biochem 37:2323–2328
Ehlers W, Gebhardt H, Meyer B (1967) K exchange and crystalIographic behaviour of three-layer clay minerals (contribution to K exchange in soil). Z Pflanzenernähr Bodenkd 117:29–53
Ehlers W, Gebhardt H, Meyer B (1968) Investigations on potassium fixation at specific sites in illite, kaolinite, montmorillonite and humus. (Contributions to K exchange in soil IV). Z Pflanzenernähr Bodenkd 119:173–186
Eischens RP (1956) Infrared spectra of chemisorbed carbon molecules. Zeitschrift Fur Elektrochemie 60:782–788
Elmaci OL, Secer M, Erdemir O, Iqbal N (2002) Ammonium fixation properties of some arable soils from the Aegean region of Turkey. Eur J Agron 17:199–208
Fan XH, Song YS, Lin DX, Yang LZ, Zhou JM (2005) Ammonia volatilization losses from urea applied to wheat on a paddy soil in Taihu Region, China. Pedosphere 15:59–65
FAO (2014) World Reference Base for soil resources 2014: international soil classification system for naming soils and creating legends for soil maps. Food and Agriculture Organization of the United Nations, Rome
Glinski J, Stahr K, Stepniewska Z, Brzezinska M (1992) Changes of redox and pH conditions in a flooded soil amended with glucose and nitrate under laboratory conditions. J Plant Nutr Soil Sc 155:13–17
Gong ZT, Chen ZC, Shi XZ et al (1999) Chinese soil taxonomy: theory. Methodology. Practice. Science Press, Beijing (in Chinese)
Han GZ, Huang LM, Tang XG (2019) Potassium supply capacity response to K-bearing mineral changes in Chinese purple paddy soil chronosequences. J Soils Sediments 19:1190–1200
Han GZ, Zhang GL (2013) Changes in magnetic properties and their pedogenetic implications for paddy soil chronosequences from different parent materials in South China. Eur J Soil Sci 64:435–444
Han GZ, Zhang GL, Li DC, Yang JL (2015) Pedogenetic evolution of clay minerals and agricultural implications for paddy soil chronosequences from different parent materials in South China. J Soils Sediments 15:423–435
Han XR, Chen EF, Guo PC, Zou DY (1999) Study on fertilizer nitrogen fixation in clay minerals and change in Brown soil. Chin J Soil Sci 30:108–109 (in Chinese)
Huang CH, Sun XF, Wang CQ, Feng WQ, Qin YS, Tu SH (2008) Study on the dynamics of nitrogen in surface water of paddy field as affected by different splitting application ratio of urea. Southw China J Agric Sci 21:1019–1022 (in Chinese)
Huang LM, Thompson A, Zhang GL (2014) Long-term paddy cultivation significantly alters topsoil phosphorus transformation and degrades phosphorus sorption capacity. Soil Tillage Res 142:32–41
Huang LM, Thompson A, Zhang GL, Chen LM, Han GZ, Gong ZT (2015) The use of chronosequence in studies of paddy soil evolution: a review. Geoderma 238:199–210
Intergovernmental Panel on Climate Change (2001) The scientific basis. In: Houghton JT et al (eds) Climate change 2001. Cambridge University Press, Cambridge, pp 7–76
Ji XH, Zheng SX, Lu YH, Liao YL (2006) Dynamics of floodwater nitrogen and its runoff loss, urea and controlled release nitrogen fertilizer application regulation in rice. Sci Agric Sin 39:2521–2530 (in Chinese)
Kaneda K (2007) Cation-exchanged montmorillonites as solid acid catalysts for organic synthesis. Synlett 21:999–1015
Keerthisinghe G, De Datta SK, Mengel K (1985) Importance of exchangeable and nonexchangeable soil NH4+ in nitrogen nutrition of lowland rice. Soil Sci 140:194–202
Kithome M, Paul JW, Lavkulich LM, Bomke AA (1998) Kinetics of ammonium adsorption and desorption by the natural zeolite clinoptilolite. Soil Sci Soc Am J 62:622–629
Lanson B, Besson G (1992) Characterization of the end of smectite-toillite transformation: decomposition of x-ray patterns. Clay Clay Miner 40:40–52
Li QK (1992) Paddy soils of China. Science Press, Beijing (in Chinese)
Liu YJ, Laird DA, Barak P (1997) Release and fixation of ammonium and potassium under long-term fertility management. Soil Sci Soc Am J 61:310–314
Liu YL, Zhang B, Li CL, Hu F, Velde B (2008) Long-term fertilization influences on clay mineral composition and ammonium adsorption in a rice paddy soil. Soil Sci Soc Am J 72:1580–1590
Mengel K, Kirkby EA (1980) Potassium in crop production. Adv Agron 33:59–110
Moore DM, Reynolds RC (1997) X-ray diffraction and the identification and analysis of clay minerals. Oxford University Press, New York
Nelson DW, Sommers LE (1982) Total carbon, organic carbon and organic matter. In: Page AL (ed) Methods of soil analysis, part 2. Chemical and microbiological properties, 2nd edn. ASA and SSSA, Madison, pp 539–579
Panaullah GM, Timsina J, Saleque MA, Ishaque M, Pathan ABMBU, Connor DJ, Saha PK, Quayyum MA, Meisner CA (2006) Nutrient uptake and apparent balances for rice-wheat sequences. III potassium. J Plant Nutr 29:173–187
Porter LK, Stewart BA (1970) Organic interferences in the fixation of ammonium by soils and clay minerals. Soil Sci 100:229–233
Regmi A, Ladha J, Pasuquin E, Pathak H, Hobbs P, Shrestha L, Gharti D, Duveiller E (2002) The role of potassium in sustaining yields in a long-term ricewheat experiment in the Indo-Gangetic Plains of Nepal. Biol Fertil Soils 36:240–247
Russell JD (1965) Infra-red study of reactions of ammonia with montmorillonite and saponite. Trans Faraday Soc 61(514P):2284–2294
Scherer HW, Zhang YS (1999) Studies on the mechanisms of fixation and release of ammonium in paddy soils after flooding: І. Effect of iron oxides on ammonium fixation. J Plant Nutr Soil Sc 162:593–597
Scherer HW, Zhang YS (2002) Mechanisms of fixation and release of ammonium in paddy soils after flooding: III effect of the oxidation state of octahedral Fe on ammonium fixation. J Plant Nutr Soil Sc 165:185–189
Schneiders M, Scherer HW (1998) Fixation and release of ammonium in flooded rice soils as affected by redox potential. Eur J Agron 8:181–189
Shen SY, Tu SI, Kemper DW (1997) Equilibrium and kinetic study of ammonium adsorption and fixation in sodium-treated vermiculite. Soil Sci Soc Am J 61:1611–1618
Velde B, Goffe B, Hoellard A (2003) Evolution of clay minerals in a chronosequence of poldered sediments under the influence of a natural pasture development. Clay Clay Miner 51:205–217
Velde B, Peck T (2002) Clay mineral changes in the Morrow experimental plots. University of Illinois. Clay Clay Miner 50:364–370
Wang XZ, Zhu JG, Hosen Y, Feng K (2004) Dynamic changes and modeling of nitrogen in paddy field surface water after application with different doses of urea. J Agro-Environ Sci 23:852–856 (in Chinese)
Whittig LD, Allardice WR (1986) X-ray diffraction techniques. In: Methods of Soil Analysis Part 1, 2nd edn. American Society of Agronomy, Inc. and Soil Science Society of America, Inc, Madison
Xie P, Jiang JM, Hseung Y (1988) Characteristics of ammonium adsorption by colloids of some main soils in China. Acta Pedol Sin 25:175–183 (in Chinese)
Zhang GL, Gong ZT (2012) Soil survey laboratory methods. Science Press, Beijing (in Chinese)
Zhang YS, Scherer HW (1999) Ammonium fixation by clay minerals in different layers of two paddy soils after flooding. Biol Fertil Soils 29:152–156
Zhang YS, Scherer HW (2000) Mechanisms of fixation and release of ammonium in paddy soils after flooding: II. Effect of transformation of nitrogen forms on ammonium fixation. Biol Fertil Soils 31:517–521
Zhang YZ, Liao JP, Sun YH, Feng YH, Huang YX (2003) Fixed ammonium in major types of paddy soils in Hunan Province, China. Pedosphere 13:199–208
Acknowledgments
The authors are grateful to Dr. David Rossiter, University of Twente, the Netherlands, for his comments and linguistic corrections.
Funding
This research was supported by the Sichuan Science and Technology Program (2018JY0527), Key Achievement Conversion Projects in Neijiang Normal University (17CZ03), Bingwei Excellent Tallents Program of Institute of Geographic Sciences and Natural Resources Research, Chinese Academy of Sciences (2017RC203), and Science and Technology Cooperation Project of Guizhou Province (Qian Ke He LH [2015]7051).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Jianming Xue
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Han, GZ., Huang, LM., Zhang, GL. et al. A chronosequence study of purple paddy soils with respect to improving ammonium and potassium fertilization management. J Soils Sediments 20, 2031–2042 (2020). https://doi.org/10.1007/s11368-019-02542-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-019-02542-w