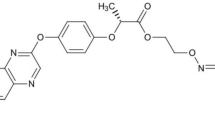
Abstract
Purpose
After the application of herbicides, their migration and transformation in the environment are closely related to their adsorption characteristics in the soil. The adsorption of herbicides in soil is also important when evaluating their environmental behaviors or risks. Pyraclonil is a contact herbicide widely used to control weeds in paddy fields, especially in Japan. This study investigated the adsorption and desorption behaviors of pyraclonil in eight typical agricultural soils across China.
Materials and methods
Surface soil samples (0–15 cm) were collected in farmland across China, including Hebei luvisols, Heilongjiang phaeozems, Sichuan gleysols, Zhejiang anthrosols, Jiangxi ferralsols, Shandong alisols, Hubei lixisols, and Hainan plinthosols. Adsorption-desorption experiments were conducted at 25 °C ± 1 °C using a batch equilibration method. Pyraclonil residue was analyzed using high-performance liquid chromatography-mass spectrometry.
Results and discussion
The adsorption and desorption isotherms of pyraclonil in the eight soils are fitted well by mathematical models. The isothermal adsorption-desorption curves of pyraclonil agree with the simulation results of the Freundlich model. The adsorption of pyraclonil is mainly physical, and all adsorption isotherms are of type L. The adsorption constants range from 3.2 to 28.6 mg1−1/n L1/n kg−1. The desorption curves of pyraclonil in all soils show positive hysteresis, as the hysteresis coefficients are in the range of 0.01–0.26. The adsorption and desorption constants increase with increasing soil organic matter content and cation exchange capacity, but they are not related to soil pH and clay content.
Conclusions
Pyraclonil exhibits low adsorption in different agricultural soils, and the adsorption process is irreversible. Pyraclonil has moderate or high mobility in different agricultural soils (except phaeozems with high organic matter content) across China, and pyraclonil could be transported from soil to groundwater after its application. Therefore, pyraclonil poses some risks to surface water and groundwater.



Similar content being viewed by others
References
Anastopoulos I, Kyzas GZ (2016) Are the thermodynamic parameters correctly estimated in liquid-phase adsorption phenomena? J Mol Liq 218:174–185
Barriuso E, Laird DA, Koskinen WC, Dowdy RH (1994) Atrazine desorption from smectites. Soil Sci Soc Am J 58:1632–1638
Browne DL, Vivat JF, Plant A, Gomez-Bengoa E, Harrity JP (2009) Investigation of the scope and regiochemistry of alkynylboronate cycloadditions with sydnones. J Am Chem Soc 131:7762–7769
Calvet R (1989) Adsorption of organic chemicals in soils. Environ Health Perspect 83:145–177
Carter MC, Kilduff JE, Weber WJ (1995) Site energy distribution analysis of preloaded adsorbents. Environ Sci Technol 29:1773–1780
Chen X, Meng Z, Yueyi S, Zhang Q, Ren L, Guan L, Ren Y, Fan T, Shen D, Yang Y (2018) Adsorption and desorption behaviors of spirotetramat in various soils and its interaction mechanism. J Agric Food Chem 66:12471–12478
Coquet Y (2003) Variation of pesticide sorption isotherm in soil at the catchment scale. Pest Manag Sci 59:69–78
Cox L, Koskinen WC, Yen PY (1998) Influence of soil properties on sorptionâ-desorption of imidacloprid. J Environ Sci Health B 33:123–134
Dorfmeister G, Franke H, Geisler J, Hartfiel U, Bohner J, Rees R (1998) New substituted pyrazole derivatives, processes for their preparation and their use as herbicides. WO, 9408999. 1994-04-28
FAO-Unesco ISSS-ISRIC (1998) World reference base of soil resources. World Soil Resources Report 84, FAO, Rome
FAO-Unesco-ISRIC(1988, 1990) Revised legend of the soil map of the world. World Soil Resouces Report No 60, FAO, Rome
Giles CH, MacEwan TH, Nakhwa SN, Smith D (1960) Studies in adsorption. Part XI. A system of classification of solution adsorption isotherms, and its use in diagnosis of adsorption mechanisms and in measurement of specific surface areas of solids. J Chem Soc 10:3973–3993
Han L, Ge Q, Mei J, Cui Y, Xue Y, Yu Y, Fang H (2019) Adsorption and desorption of carbendazim and thiamethoxam in five different agricultural soils. B Environ Contam Tox 102:550–554
Helling CS, Chesters G, Corey RB (1964) Contribution of organic matter and clay to soil cation-exchange capacity as affected by the pH of the saturating solution 1. Soil Sci Soc Am Proc 28(4):517–520
Hu M, Chen W, Liu Y, Zhang D, Chen A, Liu J, Luo X, Yan Q (2017) Determination of herbicide pyraclonil residue in rice, soil and water using high-performance liquid chromatography/tandem mass spectrometry. Anal Methods-UK 9:4790–4796
Jackson ML (1958) Soil chemical analysis. Pretice-Hall, Inc. Englewood Cliffs, NewJersey, USA
Jackson ML (1971) Soil chemical analysis. Prentice Hall Inc, Englewood Cliffs
Jin X, Ren J, Wang B, Lu Q, Yu Y, Jin X, Ren J, Wang B, Lu Q (2013) Impact of coexistence of carbendazim, atrazine, and imidacloprid on their adsorption, desorption, and mobility in soil. Environ Sci Pollut Res 20:6282–6289
Jousseaume C, Carrasco CS, Mann R, Sorribas AM (2012) Synergistic herbicidal composition containing penoxsulam and oxyfluorfen. Energy Procedia 16:687–692
Kaur P, Makkar A, Kaur P, Shilpa (2018) Temperature dependent adsorption-desorption behaviour of pendimethalin in punjab soils. Bull Environ Contam Toxicol 100:167-175
Li K, Liu W, Ying Z, Wang H (2003) Factors dominating the sorption of bentazon in soils. Chinese J Environ Sci 24:126 (in Chinese with English abstract)
Li Y, Su P, Li Y, Wen K, Bi G, Cox M (2018) Adsorption-desorption and degradation of insecticides clothianidin and thiamethoxam in agricultural soils. Chemosphere 207:708–714
Liu Y, Lonappan L, Brar SK, Yang S (2018) Impact of biochar amendment in agricultural soils on the sorption, desorption, and degradation of pesticides: a review. Sci Total Environ 645:60–70
Mader BT, Uwe-Goss K, Eisenreich SJ (1997) Sorption of nonionic, hydrophobic organic chemicals to mineral surfaces. Environ Sci Technol 31:1079–1086
Martins JM, Mermoud A (1998) Sorption and degradation of four nitroaromatic herbicides in mono and multi-solute saturated/unsaturated soil batch systems. J Contam Hydrol 33:187–210
Mikata K, Ohta K, Tashiro S (2000) Adsorption and desorption of herbicide imazosulfuron in soils. J Pestic Sci 25:212–216
Oliver DP, Kookana RS, Quintana B (2005) Sorption of pesticides in tropical and temperate soils from australia and the Philippines. J Agric Food Chem 53:6420–6425
Organisation for Economic Co-operation and Development (2000) OECD guideline for the testing of chemicals: adsorption-desorption using a batch equilibrium method. (Updated Guideline, adopted 21st January 2000)
Piper CS (2011) Soil and plant analysis. Scientific Publishers, Jodhpur
Pusino A, Pinna MV, Gessa C (2004) Azimsulfuron sorption−desorption on soil. J Agric Food Chem 52:3462–3466
Rae JE, Cooper CS, Parker A, Peters A (1998) Pesticide sorption onto aquifer sediments. J Geochem Explor 64:263–276
Toan PV, Sebesvari Z, Bläsing M, Rosendahl I, Renaud FG (2013) Pesticide management and their residues in sediments and surface and drinking water in the Mekong Delta, Vietnam. Sci Total Environ 452-453:28–39
Tucker BV, Pack DE, Ospenson JN (1967) Adsorption of bipyridylium herbicides in soil. J Agric Food Chem 15:1005–1008
Walkly A (1934) An examination of the Degtjareff method for determining soil organic matter and a proposed modification to the chromic acid titration method. Soil Sci 37:29–38
Wang Q, University Z, Hangzhou (1998) Adsorption and desorption of herbicide imazapyr by soil. China Environ Sci 18:314–318 (in Chinese with English abstract)
Wang Z, Cang T, Qi P, Zhao X, Xu H, Wang X, Zhang H, Wang X (2015) Dissipation of four fungicides on greenhouse strawberries and an assessment of their risks. Food Control 55:215–220
Westra EP (2012) Adsorption, leaching, and dissipation of pyroxasulfone and two chloroacetamide herbicides. MSc. thesis. Fort Collins, CO: Colorado State University
Yu L (2009) Is the free energy change of adsorption correctly calculated? J Chem Eng Data 54(7):1981–1985
Zhang YB (2014) Development and application of pyraclonil in paddy field. World Pestic 36:1–3 (in Chinese with English abstract)
Zhang W, Wang JJ, Zhang ZM, Qin Z (2006) Adsorption of nicosulfuron on soils and its correlation with soil properties. Chinese J Pestic Sci 8:265–271
Zhang W, Wang JJ, Zhang ZM, Qin Z (2007) Adsorption-desorption characteristics of chlorimuron-ethyl in soils. Agric Sci China 6:1359–1368 (in Chinese with English abstract)
Funding
This work is financially supported by the National Key Research and Development Plan of China (Grant No. 2017YFD0301604) and the Science and Technology Project of Jiangxi Education Department (Grant No. GJJ170310).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Claudio Colombo
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, Y., Li, W., Zhou, W. et al. Adsorption-desorption characteristics of pyraclonil in eight agricultural soils. J Soils Sediments 20, 1404–1412 (2020). https://doi.org/10.1007/s11368-019-02471-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-019-02471-8