Abstract
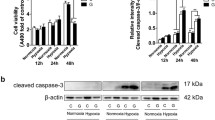
Diabetes increases the risk of Alzheimer’s disease (AD). We investigated the impact of glucose concentrations on the β-amyloid (Aβ)-induced alteration of mitochondrial/cellular energetics in primary human brain microvascular endothelial cells (HBMECs). HBMECs were grown and passaged in media containing 15 mmol/l glucose (normal) based on which the glucose levels in the media were designated as high (25 mmol/L) or low (5 mmol/L). HBMECs were treated with Aβ (1–42) (5 µmol/l) or a scrambled peptide for 24 h and mitochondrial respiratory parameters were measured using Seahorse Mito Stress Test. Aβ (1–42) decreased the mitochondrial ATP production at normal glucose levels and decreased spare respiratory capacity at high glucose levels. Aβ (1–42) diminished all mitochondrial respiratory parameters markedly at low glucose levels that were not completely recovered by restoring normal glucose levels in the media. The addition of mannitol (10 mmol/l) to low and normal glucose-containing media altered the Aβ (1–42)-induced bioenergetic defects. Even at normal glucose levels, pre-senescent HMBECs (passage 15) displayed greater Aβ (1–42)-induced mitochondrial respiratory impairments than young cells (passages 7–9). Thus, hypoglycemia, osmolarity changes, and senescence are stronger instigators of Aβ (1–42)-induced mitochondrial respiration and energetics in HBMECs and contributors to diabetes-related increased AD risk than hyperglycemia.







Similar content being viewed by others
References
Kumar A, Singh A and Ekavali. A review on Alzheimer’s disease pathophysiology and its management: an update. Pharmacol Rep 2015; 67: 195–203. 20140922. https://doi.org/10.1016/j.pharep.2014.09.004.
Vagelatos NT, Eslick GD. Type 2 diabetes as a risk factor for Alzheimer’s disease: the confounders, interactions, and neuropathology associated with this relationship. Epidemiol Rev. 2013;35:152–60. https://doi.org/10.1093/epirev/mxs012.
Chatterjee S and Mudher A. Alzheimer’s disease and type 2 diabetes: a critical assessment of the shared pathological traits. Front Neurosci 2018; 12. Review. https://doi.org/10.3389/fnins.2018.00383.
Aa R. Risk factors for Alzheimer’s disease. Folia Neuropathol. 2019;57:87–105. https://doi.org/10.5114/fn.2019.85929.
He C, Li Q, Cui Y, Gao P, Shu W, Zhou Q, Wang L, Li L, Lu Z, Zhao Y, Ma H, Chen X, Jia H, Zheng H, Yang G, Liu D, Tepel M and Zhu Z. Recurrent moderate hypoglycemia accelerates the progression of Alzheimer’s disease through impairment of the TRPC6/GLUT3 pathway. JCI Insight 2022; 7 20220308. https://doi.org/10.1172/jci.insight.154595.
Rhee SY. Hypoglycemia and dementia. Endocrinol Metab (Seoul). 2017;32:195–9. https://doi.org/10.3803/EnM.2017.32.2.195.
Steinman J, Sun H-S and Feng Z-P. Microvascular alterations in Alzheimer’s disease. Front Cell Neurosci 2021; 14. Review. https://doi.org/10.3389/fncel.2020.618986.
Scheffer S, Hermkens DMA, Weerd Lvd, Vries HEd and Daemen MJAP. Vascular hypothesis of Alzheimer disease. Arterioscler Thromb Vasc Biol 2021; 41: 1265–1283. https://doi.org/10.1161/ATVBAHA.120.311911.
Klohs J. An integrated view on vascular dysfunction in Alzheimer’s disease. Neurodegener Dis. 2019;19:109–27. https://doi.org/10.1159/000505625.
van Sloten TT, Sedaghat S, Carnethon MR, Launer LJ, Stehouwer CDA. Cerebral microvascular complications of type 2 diabetes: stroke, cognitive dysfunction, and depression. Lancet Diabetes Endocrinol. 2020;8(325–336):20200302. https://doi.org/10.1016/s2213-8587(19)30405-x.
Wang D, Chen F, Han Z, Yin Z, Ge X and Lei P. Relationship between amyloid-β deposition and blood–brain barrier dysfunction in Alzheimer’s disease. Front Cell Neurosci 2021; 15. Review. https://doi.org/10.3389/fncel.2021.695479.
Vadukul DM, Gbajumo O, Marshall KE, Serpell LC. Amyloidogenicity and toxicity of the reverse and scrambled variants of amyloid-β 1–42. FEBS Lett. 2017;591:822–30. https://doi.org/10.1002/1873-3468.12590.
Roher AE, Lowenson JD, Clarke S, Woods AS, Cotter RJ, Gowing E, Ball MJ. beta-Amyloid-(1–42) is a major component of cerebrovascular amyloid deposits: implications for the pathology of Alzheimer disease. Proc Natl Acad Sci U S A. 1993;90:10836–40. https://doi.org/10.1073/pnas.90.22.10836.
Hampel H, Hardy J, Blennow K, Chen C, Perry G, Kim SH, Villemagne VL, Aisen P, Vendruscolo M, Iwatsubo T, Masters CL, Cho M, Lannfelt L, Cummings JL, Vergallo A. The amyloid-β pathway in Alzheimer’s disease. Mol Psychiatry. 2021;26:5481–503. https://doi.org/10.1038/s41380-021-01249-0.
Sotthibundhu A, Sykes AM, Fox B, Underwood CK, Thangnipon W, Coulson EJ. Beta-amyloid(1–42) induces neuronal death through the p75 neurotrophin receptor. J Neurosci. 2008;28:3941–6. https://doi.org/10.1523/jneurosci.0350-08.2008.
Allaman I, Gavillet M, Bélanger M, Laroche T, Viertl D, Lashuel HA, Magistretti PJ. Amyloid-beta aggregates cause alterations of astrocytic metabolic phenotype: impact on neuronal viability. J Neurosci. 2010;30:3326–38. https://doi.org/10.1523/jneurosci.5098-09.2010.
Jana M, Palencia CA, Pahan K. Fibrillar amyloid-β peptides activate microglia via TLR2: implications for Alzheimer’s disease. J Immunol. 2008;181:7254–62. https://doi.org/10.4049/jimmunol.181.10.7254.
Yue Q, Zhou X, Zhang Z and Hoi MPM. Murine Beta-Amyloid (1–42) Oligomers disrupt endothelial barrier integrity and VEGFR signaling via activating astrocytes to release deleterious soluble factors. Int J Mol Sci 2022; 23 20220207. https://doi.org/10.3390/ijms23031878.
Alcendor DJ. Interactions between amyloid-Β proteins and human brain pericytes: implications for the pathobiology of Alzheimer’s disease. J Clin Med 2020; 9 20200515. DOI: https://doi.org/10.3390/jcm9051490.
Chen JX, Yan SD. Amyloid-beta-induced mitochondrial dysfunction. J Alzheimers Dis. 2007;12:177–84. https://doi.org/10.3233/jad-2007-12208.
Yao Y, Huang JZ, Chen Y, Hu HJ, Tang X, Li X. Effects and mechanism of amyloid β1-42 on mitochondria in astrocytes. Mol Med Rep. 2018;17(6997–7004):20180316. https://doi.org/10.3892/mmr.2018.8761.
Marco S, Skaper SD. Amyloid beta-peptide1-42 alters tight junction protein distribution and expression in brain microvessel endothelial cells. Neurosci Lett. 2006;401(219–224):20060427. https://doi.org/10.1016/j.neulet.2006.03.047.
Singh Angom R, Wang Y, Wang E, Pal K, Bhattacharya S, Watzlawik JO, Rosenberry TL, Das P, Mukhopadhyay D. VEGF receptor-1 modulates amyloid β 1–42 oligomer-induced senescence in brain endothelial cells. Faseb j. 2019;33(4626–4637):20181221. https://doi.org/10.1096/fj.201802003R.
Park R, Kook SY, Park JC, Mook-Jung I. Aβ1–42 reduces P-glycoprotein in the blood–brain barrier through RAGE–NF-κB signaling. Cell Death Dis. 2014;5:e1299–e1299. https://doi.org/10.1038/cddis.2014.258.
Quintana DD, Garcia JA, Anantula Y, Rellick SL, Engler-Chiurazzi EB, Sarkar SN, Brown CM, Simpkins JW. Amyloid-β causes mitochondrial dysfunction via a Ca2+-driven upregulation of oxidative phosphorylation and superoxide production in cerebrovascular endothelial cells. J Alzheimers Dis. 2020;75:119–38. https://doi.org/10.3233/jad-190964.
Doll DN, Hu H, Sun J, Lewis SE, Simpkins JW, Ren X. Mitochondrial crisis in cerebrovascular endothelial cells opens the blood-brain barrier. Stroke. 2015;46(1681–1689):20150428. https://doi.org/10.1161/strokeaha.115.009099.
Kluge MA, Fetterman JL, Vita JA. Mitochondria and endothelial function. Circ Res. 2013;112:1171–88. https://doi.org/10.1161/circresaha.111.300233.
Caja S, Enríquez JA. Mitochondria in endothelial cells: sensors and integrators of environmental cues. Redox Biol. 2017;12(821–827):20170418. https://doi.org/10.1016/j.redox.2017.04.021.
Sakamuri S, Sure VN, Kolli L, Liu N, Evans WR, Sperling JA, Busija DW, Wang X, Lindsey SH, Murfee WL, Mostany R and Katakam PVG. Glycolytic and oxidative phosphorylation defects precede the development of senescence in primary human brain microvascular endothelial cells. Geroscience 2022 2022/04/06. https://doi.org/10.1007/s11357-022-00550-2.
Sakamuri SS, Sure VN, Kolli L, Evans WR, Sperling JA, Bix GJ, Wang X, Atochin DN, Murfee WL, Mostany R and Katakam PV. Aging related impairment of brain microvascular bioenergetics involves oxidative phosphorylation and glycolytic pathways. J Cereb Blood Flow Metab; 0: 0271678X211069266. https://doi.org/10.1177/0271678x211069266.
Parodi-Rullán R, Sone JY and Fossati S. Endothelial mitochondrial dysfunction in cerebral amyloid angiopathy and Alzheimer’s disease. J Alzheimers Dis 2019; 72: 1019–1039. https://doi.org/10.3233/jad-190357
Kim DK and Mook-Jung I. The role of cell type-specific mitochondrial dysfunction in the pathogenesis of Alzheimer’s disease. BMB Rep 2019; 52: 679–688. https://doi.org/10.5483/BMBRep.2019.52.12.282.
Rehni AK, Nautiyal N, Perez-Pinzon MA and Dave KR. Hyperglycemia / hypoglycemia-induced mitochondrial dysfunction and cerebral ischemic damage in diabetics. Metab Brain Dis 2015; 30: 437–447. https://doi.org/10.1007/s11011-014-9538-z.
Lamoke F, Mazzone V, Persichini T, Maraschi A, Harris MB, Venema RC, Colasanti M, Gliozzi M, Muscoli C, Bartoli M, Mollace V. Amyloid β peptide-induced inhibition of endothelial nitric oxide production involves oxidative stress-mediated constitutive eNOS/HSP90 interaction and disruption of agonist-mediated Akt activation. J Neuroinflammation. 2015;12(84):20150503. https://doi.org/10.1186/s12974-015-0304-x.
Kook SY, Hong HS, Moon M, Ha CM, Chang S, Mook-Jung I. Aβ1-42-RAGE interaction disrupts tight junctions of the blood-brain barrier via Ca2+-calcineurin signaling. J Neurosci. 2012;32:8845–54. https://doi.org/10.1523/jneurosci.6102-11.2012.
Cenini G, Voos W. Mitochondria as potential targets in alzheimer disease therapy: an update. Front Pharmacol. 2019;10(902):20190823. https://doi.org/10.3389/fphar.2019.00902.
Abramov AY, Duchen MR. The role of an astrocytic NADPH oxidase in the neurotoxicity of amyloid beta peptides. Philos Trans R Soc Lond B Biol Sci. 2005;360:2309–14. https://doi.org/10.1098/rstb.2005.1766.
Akhtar MW, Sanz-Blasco S, Dolatabadi N, Parker J, Chon K, Lee MS, Soussou W, McKercher SR, Ambasudhan R, Nakamura T, Lipton SA. Elevated glucose and oligomeric β-amyloid disrupt synapses via a common pathway of aberrant protein S-nitrosylation. Nat Commun. 2016;7(10242):20160108. https://doi.org/10.1038/ncomms10242.
Prasad S, Sajja RK, Naik P, Cucullo L. Diabetes mellitus and blood-brain barrier dysfunction: an overview. J Pharmacovigil. 2014;2:125. https://doi.org/10.4172/2329-6887.1000125.
Macauley SL, Stanley M, Caesar EE, Yamada SA, Raichle ME, Perez R, Mahan TE, Sutphen CL, Holtzman DM. Hyperglycemia modulates extracellular amyloid-β concentrations and neuronal activity in vivo. J Clin Invest. 2015;125(2463–2467):20150504. https://doi.org/10.1172/jci79742.
Chao AC, Lee TC, Juo SH, Yang DI. Hyperglycemia increases the production of amyloid beta-peptide leading to decreased endothelial tight junction. CNS Neurosci Ther. 2016;22(291–297):20160204. https://doi.org/10.1111/cns.12503.
Yan LJ. Pathogenesis of chronic hyperglycemia: from reductive stress to oxidative stress. J Diabetes Res. 2014;2014(137919):20140616. https://doi.org/10.1155/2014/137919.
Shafiee G, Mohajeri-Tehrani M, Pajouhi M, Larijani B. The importance of hypoglycemia in diabetic patients. J Diabetes Metab Disord. 2012;11(17):20121001. https://doi.org/10.1186/2251-6581-11-17.
Han E, Han KD, Lee BW, Kang ES, Cha BS, Ko SH, Lee YH. Severe hypoglycemia increases dementia risk and related mortality: a nationwide, population-based cohort study. J Clin Endocrinol Metab. 2022;107:e1976–86. https://doi.org/10.1210/clinem/dgab860.
Kim YG, Park DG, Moon SY, Jeon JY, Kim HJ, Kim DJ, Lee KW, Han SJ. Hypoglycemia and dementia risk in older patients with type 2 diabetes mellitus: a propensity-score matched analysis of a population-based cohort study. Diabetes Metab J. 2020;44(125–133):20191023. https://doi.org/10.4093/dmj.2018.0260.
Lee CW, Shih YH, Wu SY, Yang T, Lin C, Kuo YM. Hypoglycemia induces tau hyperphosphorylation. Curr Alzheimer Res. 2013;10:298–308. https://doi.org/10.2174/1567205011310030009.
Moin ASM, Al-Qaissi A, Sathyapalan T, Atkin SL, Butler AE. Hypoglycaemia in type 2 diabetes exacerbates amyloid-related proteins associated with dementia. Diabetes Obes Metab. 2021;23(338–349):20201025. https://doi.org/10.1111/dom.14220.
Shi J, Xiang Y, Simpkins JW. Hypoglycemia enhances the expression of mRNA encoding beta-amyloid precursor protein in rat primary cortical astroglial cells. Brain Res. 1997;772:247–51. https://doi.org/10.1016/s0006-8993(97)00827-5.
Jung HJ, Kim YJ, Eggert S, Chung KC, Choi KS, Park SA. Age-dependent increases in tau phosphorylation in the brains of type 2 diabetic rats correlate with a reduced expression of p62. Exp Neurol. 2013;248(441–450):20130729. https://doi.org/10.1016/j.expneurol.2013.07.013.
Lewis H, Beher D, Cookson N, Oakley A, Piggott M, Morris CM, Jaros E, Perry R, Ince P, Kenny RA, Ballard CG, Shearman MS, Kalaria RN. Quantification of Alzheimer pathology in ageing and dementia: age-related accumulation of amyloid-beta(42) peptide in vascular dementia. Neuropathol Appl Neurobiol. 2006;32:103–18. https://doi.org/10.1111/j.1365-2990.2006.00696.x.
Ungureanu AA, Benilova I, Krylychkina O, Braeken D, De Strooper B, Van Haesendonck C, Dotti CG, Bartic C. Amyloid beta oligomers induce neuronal elasticity changes in age-dependent manner: a force spectroscopy study on living hippocampal neurons. Sci Rep. 2016;6(25841):20160513. https://doi.org/10.1038/srep25841.
Kulkarni T, Angom RS, Das P, Bhattacharya S, Mukhopadhyay D. Nanomechanical insights: amyloid beta oligomer-induced senescent brain endothelial cells. Biochim Biophys Acta Biomembr. 2019;1861(183061):20190909. https://doi.org/10.1016/j.bbamem.2019.183061.
Sakamuri SS, Sure VN, Kolli L, Evans WR, Sperling JA, Bix GJ, Wang X, Atochin DN, Murfee WL, Mostany R and Katakam PV. Aging related impairment of brain microvascular bioenergetics involves oxidative phosphorylation and glycolytic pathways. J Cereb Blood Flow Metab 2022: 271678X211069266. https://doi.org/10.1177/0271678X211069266.
Kiss T, Balasubramanian P, Valcarcel-Ares MN, Tarantini S, Yabluchanskiy A, Csipo T, Lipecz A, Reglodi D, Zhang XA, Bari F, Farkas E, Csiszar A and Ungvari Z. Nicotinamide mononucleotide (NMN) treatment attenuates oxidative stress and rescues angiogenic capacity in aged cerebromicrovascular endothelial cells: a potential mechanism for the prevention of vascular cognitive impairment. Geroscience 2019; 41: 619–630. https://doi.org/10.1007/s11357-019-00074-2.
Domoki F, Kis B, Gaspar T, Bari F and Busija DW. Cerebromicrovascular endothelial cells are resistant to L-glutamate. Am J Physiol Regul Integr Comp Physiol 2008; 295: R1099–1108. https://doi.org/10.1152/ajpregu.90430.2008.
Kis B, Snipes JA, Simandle SA and Busija DW. Acetaminophen-sensitive prostaglandin production in rat cerebral endothelial cells. Am J Physiol Regul Integr Comp Physiol 2005; 288: R897–902. https://doi.org/10.1152/ajpregu.00613.2004.
Huang Y, Xiong ZG. Choosing an appropriate glucose concentration according to different cell types and experimental purposes is very important. Cell Stress Chaperones. 2015;20(1–2):20141010. https://doi.org/10.1007/s12192-014-0547-y.
Funding
This research project was supported by the National Institutes of Health: National Institute of Neurological Disorders and Stroke (NS094834 and NS114286—P.V. Katakam; NS114286 – R. Mostany; NS099539 – X. Wang) and National Institute on Aging (AG047296 – R. Mostany; AG074489 – P.V. Katakam and R. Mostany). In addition, the study was supported by American Heart Association (National Center Scientist Development Grant, 14SDG20490359—P.V. Katakam; Greater Southeast Affiliate Predoctoral Fellowship Award, 16PRE27790122—V.N. Sure).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Sakamuri, S.S.V.P., Sure, V.N., Wang, X. et al. Amyloid \(\upbeta\) (1–42) peptide impairs mitochondrial respiration in primary human brain microvascular endothelial cells: impact of dysglycemia and pre-senescence. GeroScience 44, 2721–2739 (2022). https://doi.org/10.1007/s11357-022-00644-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-022-00644-x