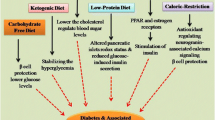
Abstract
The field of aging research has grown rapidly over the last half-century, with advancement of scientific technologies to interrogate mechanisms underlying the benefit of life-extending interventions like calorie restriction (CR). Coincident with this increase in knowledge has been the rise of obesity and type 2 diabetes (T2D), both associated with increased morbidity and mortality. Given the difficulty in practicing long-term CR, a search for compounds (CR mimetics) which could recapitulate the health and longevity benefits without requiring food intake reductions was proposed. Alpha-glucosidase inhibitors (AGIs) are compounds that function predominantly within the gastrointestinal tract to inhibit α-glucosidase and α-amylase enzymatic digestion of complex carbohydrates, delaying and decreasing monosaccharide uptake from the gut in the treatment of T2D. Acarbose, an AGI, has been shown in pre-clinical models to increase lifespan (greater longevity benefits in males), with decreased body weight gain independent of calorie intake reduction. The CR mimetic benefits of acarbose are further supported by clinical findings beyond T2D including the risk for other age-related diseases (e.g., cancer, cardiovascular). Open questions remain regarding the exclusivity of acarbose relative to other AGIs, potential off-target effects, and combination with other therapies for healthy aging and longevity extension. Given the promising results in pre-clinical models (even in the absence of T2D), a unique mechanism of action and multiple age-related reduced disease risks that have been reported with acarbose, support for clinical trials with acarbose focusing on aging-related outcomes and incorporating biological sex, age at treatment initiation, and T2D-dependence within the design is warranted.

Similar content being viewed by others
References
Weindruch R, Walford RL, The retardation of aging and disease by dietary restriction. Springfield. IL: C. C. Thomas Publisher; 1988.
Masoro EJ. Dietary restriction: current status. Aging (Milano). 2001;13(4):261–2.
Masoro EJ. Dietary restriction-induced life extension: a broadly based biological phenomenon. Biogerontology. 2006;7(3):153–5.
McCay CM. Effect of restricted feeding upon aging and chronic diseases in rats and dogs. Am J Public Health Nations Health. 1947;37(5):521–8.
McCay CM, Pope F, Lunsford W. Experimental prolongation of the life span. Bull N Y Acad Med. 1956;32(2):91–101.
Masoro EJ. Caloric restriction-induced life extension of rats and mice: a critique of proposed mechanisms. Biochim Biophys Acta. 2009;1790(10):1040–8.
Weindruch R, Walford RL, Fligiel S, Guthrie D. The retardation of aging in mice by dietary restriction: longevity, cancer, immunity and lifetime energy intake. J Nutr. 1986;116(4):641–54.
Hursting SD, Lavigne JA, Berrigan D, Perkins SN, Barrett JC. Calorie restriction, aging, and cancer prevention: mechanisms of action and applicability to humans. Annu Rev Med. 2003;54:131–52.
Everitt AV, Le Couteur DG. Life extension by calorie restriction in humans. Ann N Y Acad Sci. 2007;1114:428–33.
Fontana L. Calorie restriction and cardiometabolic health. Eur J Cardiovasc Prev Rehabil. 2008;15(1):3–9.
Fontana L. Nutrition, adiposity and health. Epidemiol Prev. 2007;31(5):290–4.
Flegal KM, Williamson DF, Pamuk ER, Rosenberg HM. Estimating deaths attributable to obesity in the United States. Am J Public Health. 2004;94(9):1486–9.
Flegal KM. Epidemiologic aspects of overweight and obesity in the United States. Physiol Behav. 2005;86(5):599–602.
Smith DL Jr, Yang Y, Nagy TR, Patki A, Vasselli JR, Zhang Y, et al. Weight cycling increases longevity compared with sustained obesity in mice. Obesity (Silver Spring). 2018;26(11):1733–9.
Ingram DK, et al. Development of calorie restriction mimetics as a prolongevity strategy. Ann N Y Acad Sci. 2004;1019:412–23.
Ingram DK, Roth GS. Glycolytic inhibition as a strategy for developing calorie restriction mimetics. Exp Gerontol. 2011;46(2-3):148–54.
Ingram DK, Roth GS. Calorie restriction mimetics: can you have your cake and eat it, too? Ageing Res Rev. 2015;20:46–62.
Sherwin R, Jastreboff AM. Year in diabetes 2012: the diabetes tsunami. J Clin Endocrinol Metab. 2012;97(12):4293–301.
Menke A, Rust KF, Fradkin J, Cheng YJ, Cowie CC. Associations between trends in race/ethnicity, aging, and body mass index with diabetes prevalence in the United States: a series of cross-sectional studies. Ann Intern Med. 2014;161(5):328–35.
Smith DL Jr, et al. Metformin supplementation and life span in Fischer-344 rats. J Gerontol A Biol Sci Med Sci. 2010;65(5):468–74.
Dhahbi JM, Mote PL, Fahy GM, Spindler SR. Identification of potential caloric restriction mimetics by microarray profiling. Physiol Genomics. 2005;23(3):343–50.
Greenway F. Obesity medications and the treatment of type 2 diabetes. Diabetes Technol Ther. 1999;1(3):277–87.
Smith DL Jr, Robertson HT, Desmond RA, Nagy TR, Allison DB. No compelling evidence that sibutramine prolongs life in rodents despite providing a dose-dependent reduction in body weight. Int J Obes. 2011;35(5):652–7.
Minor RK, Smith DL Jr, Sossong AM, Kaushik S, Poosala S, Spangler EL, et al. Chronic ingestion of 2-deoxy-D-glucose induces cardiac vacuolization and increases mortality in rats. Toxicol Appl Pharmacol. 2010;243(3):332–9.
Clissold SP, Edwards C. Acarbose. A preliminary review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential. Drugs. 1988;35(3):214–43.
Bischoff, H., Pharmacology of alpha-glucosidase inhibition. Eur J Clin Investig, 1994. 24 Suppl 3: p. 3-10.
Kritchevsky D. Caloric restriction and cancer. J Nutr Sci Vitaminol (Tokyo). 2001;47(1):13–9.
Longo VD, Fontana L. Calorie restriction and cancer prevention: metabolic and molecular mechanisms. Trends Pharmacol Sci. 2010;31(2):89–98.
Rous P. The influence of diet on transplanted and spontaneous mouse tumors. J Exp Med. 1914;20(5):433–51.
Masoro EJ. Caloric restriction and aging: an update. Exp Gerontol. 2000;35(3):299–305.
Fontana L. The scientific basis of caloric restriction leading to longer life. Curr Opin Gastroenterol. 2009;25(2):144–50.
Ogden CL, Carroll MD, Flegal KM. Epidemiologic trends in overweight and obesity. Endocrinol Metab Clin N Am. 2003;32(4):741–60 vii.
Lahey R, Khan SS. Trends in obesity and risk of cardiovascular disease. Curr Epidemiol Rep. 2018;5(3):243–51.
Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988-2012. JAMA. 2015;314(10):1021–9.
Hales CM, Fryar CD, Carroll MD, Freedman DS, Ogden CL. Trends in obesity and severe obesity prevalence in US youth and adults by sex and age, 2007-2008 to 2015-2016. JAMA. 2018;319(16):1723–5.
Hales, C.M., et al., Prevalence of obesity and severe obesity among adults: United States, 2017-2018. NCHS Data Brief, 2020(360): p. 1-8.
Brewer RA, Gibbs VK, Smith DL Jr. Targeting glucose metabolism for healthy aging. Nutr Healthy Aging. 2016;4(1):31–46.
Krause HP, Keup U, Puls W. Inhibition of disaccharide digestion in rat intestine by the alpha-glucosidase inhibitor acarbose (BAY g 5421). Digestion. 1982;23(4):232–8.
Lee SM, Bustamante SA, Koldovsky O. The effect of alpha-glucosidase inhibition on intestinal disaccharidase activity in normal and diabetic mice. Metabolism. 1983;32(8):793–9.
Balfour JA, McTavish D. Acarbose. An update of its pharmacology and therapeutic use in diabetes mellitus. Drugs. 1993;46(6):1025–54.
Jenkins DJ, et al. Scope and specificity of acarbose in slowing carbohydrate absorption in man. Diabetes. 1981;30(11):951–4.
McCulloch DK, Tattersall RB. Pharmacological fibre’--alpha-glucosidase inhibition in the management of diabetes. Diabet Med. 1984;1(3):189–90.
Jenkins DJ, Taylor RH, Nineham R, Goff DV, Bloom SR, Sarson DL, et al. Manipulation of gut hormone response to food by soluble fiber and alpha-glucosidase inhibition. Am J Gastroenterol. 1988;83(4):393–7.
Mc CCK, CC, Woodward JC, Sehgal BS. Cellulose in the diet of rats and mice. J Nutr. 1934;8(4):435–47.
Brussow H. Microbiota and healthy ageing: observational and nutritional intervention studies. Microb Biotechnol. 2013;6(4):326–34.
Tachon S, Zhou J, Keenan M, Martin R, Marco ML. The intestinal microbiota in aged mice is modulated by dietary resistant starch and correlated with improvements in host responses. FEMS Microbiol Ecol. 2013;83(2):299–309.
Keenan MJ, Marco ML, Ingram DK, Martin RJ. Improving healthspan via changes in gut microbiota and fermentation. Age (Dordr). 2015;37(5):98.
Hollander P. Safety profile of acarbose, an alpha-glucosidase inhibitor. Drugs. 1992;44(Suppl 3):47–53.
Martin AE, Montgomery PA. Acarbose: an alpha-glucosidase inhibitor. Am J Health Syst Pharm. 1996;53(19):2277–90.
Smith DL Jr, et al. Calorie restriction extends the chronological lifespan of Saccharomyces cerevisiae independently of the Sirtuins. Aging Cell. 2007;6(5):649–62.
Troen AM, French EE, Roberts JF, Selhub J, Ordovas JM, Parnell LD, et al. Lifespan modification by glucose and methionine in Drosophila melanogaster fed a chemically defined diet. Age (Dordr). 2007;29(1):29–39.
Choi SS. High glucose diets shorten lifespan of Caenorhabditis elegans via ectopic apoptosis induction. Nutr Res Pract. 2011;5(3):214–8.
Jiang JC, Jaruga E, Repnevskaya MV, Jazwinski SM. An intervention resembling caloric restriction prolongs life span and retards aging in yeast. FASEB J. 2000;14(14):2135–7.
Mlekusch W, Lamprecht M, Öttl K, Tillian M, Reibnegger G. A glucose-rich diet shortens longevity of mice. Mech Ageing Dev. 1996;92(1):43–51.
Harrison DE, Strong R, Allison DB, Ames BN, Astle CM, Atamna H, et al. Acarbose, 17-alpha-estradiol, and nordihydroguaiaretic acid extend mouse lifespan preferentially in males. Aging Cell. 2014;13(2):273–82.
Strong R, Miller RA, Antebi A, Astle CM, Bogue M, Denzel MS, et al. Longer lifespan in male mice treated with a weakly estrogenic agonist, an antioxidant, an alpha-glucosidase inhibitor or a Nrf2-inducer. Aging Cell. 2016;15(5):872–84.
Cohen AM, Rosenmann E. Acarbose treatment and diabetic nephropathy in the Cohen diabetic rat. Horm Metab Res. 1990;22(10):511–5.
Harrison DE, Strong R, Alavez S, Astle CM, DiGiovanni J, Fernandez E, et al. Acarbose improves health and lifespan in aging HET3 mice. Aging Cell. 2019;18(2):e12898.
McCarty MF, DiNicolantonio JJ. Acarbose, lente carbohydrate, and prebiotics promote metabolic health and longevity by stimulating intestinal production of GLP-1. Open Heart. 2015;2(1):e000205.
Lee SM. The effect of chronic alpha-glycosidase inhibition on diabetic nephropathy in the db/db mouse. Diabetes. 1982;31(3):249–54.
Casirola DM, Ferraris RP. alpha-Glucosidase inhibitors prevent diet-induced increases in intestinal sugar transport in diabetic mice. Metabolism. 2006;55(6):832–41.
Madariaga H, Lee PC, Heitlinger LA, Lebenthal E. Effects of graded alpha-glucosidase inhibition on sugar absorption in vivo. Dig Dis Sci. 1988;33(8):1020–4.
Madar Z. The effect of acarbose and miglitol (BAY-M-1099) on postprandial glucose levels following ingestion of various sources of starch by nondiabetic and streptozotocin-induced diabetic rats. J Nutr. 1989;119(12):2023–9.
Katovich MJ, Meldrum MJ, Vasselli JR. Beneficial effects of dietary acarbose in the streptozotocin-induced diabetic rat. Metabolism. 1991;40(12):1275–82.
Chiasson JL, Josse RG, Hunt JA, Palmason C, Rodger NW, Ross SA, et al. The efficacy of acarbose in the treatment of patients with non-insulin-dependent diabetes mellitus. A multicenter controlled clinical trial. Ann Intern Med. 1994;121(12):928–35.
Coniff R, Krol A. Acarbose: a review of US clinical experience. Clin Ther. 1997;19(1):16–26.
Chiasson JL, Josse RG, Leiter LA, Mihic M, Nathan DM, Palmason C, et al. The effect of acarbose on insulin sensitivity in subjects with impaired glucose tolerance. Diabetes Care. 1996;19(11):1190–3.
Macedo CS, Silva MD, Spadella CT, Breim LC, Capeletti S, Mercadante MC, et al. Effect of long-term treatment with insulin and/or acarbose on glomerular basement membrane thickening in alloxan-diabetic rats. Braz J Med Biol Res. 1996;29(10):1329–35.
Dimitriadis G, Tessari P, Go V, Gerich J. Effects of the disaccharidase inhibitor acarbose on meal and intravenous glucose tolerance in normal man. Metabolism. 1982;31(8):841–3.
Holt PR, Atillasoy E, Lindenbaum J, Ho SB, Lupton JR, McMahon D, et al. Effects of acarbose on fecal nutrients, colonic pH, and short-chain fatty acids and rectal proliferative indices. Metabolism. 1996;45(9):1179–87.
Brooks B, Molyneaux L, Zilkens R, Ross G, Yue DK. The use of acarbose in type 2 diabetic patients in secondary failure: effects on glycaemic control and diet induced thermogenesis. Diabetes Res Clin Pract. 1998;42(3):175–80.
Chiasson JL, et al. Acarbose for the prevention of Type 2 diabetes, hypertension and cardiovascular disease in subjects with impaired glucose tolerance: facts and interpretations concerning the critical analysis of the STOP-NIDDM Trial data. Diabetologia. 2004;47(6):969–75 discussion 976-7.
Ahr HJ, Boberg M, Krause HP, Maul W, Müller FO, Ploschke HJ, et al. Pharmacokinetics of acarbose. Part I: absorption, concentration in plasma, metabolism and excretion after single administration of [14C] acarbose to rats, dogs and man. Arzneimittelforschung. 1989;39(10):1254–60.
Haupt VJ, Daminelli S, Schroeder M. Drug Promiscuity in PDB: Protein Binding Site Similarity Is Key. PLoS One. 2013;8(6):e65894.
Gibbs VK, Brewer RA, Miyasaki ND, Patki A, Smith DL Jr. Sex-dependent differences in liver and gut metabolomic profiles with acarbose and calorie restriction in C57BL/6 mice. J Gerontol A Biol Sci Med Sci. 2018;73(2):157–65.
Su B, Liu H, Li J, Sunli Y, Liu B, Liu D, et al. Acarbose treatment affects the serum levels of inflammatory cytokines and the gut content of bifidobacteria in Chinese patients with type 2 diabetes mellitus. J Diabetes. 2015;7(5):729–39.
Zhang M, Feng R, Yang M, Qian C, Wang Z, Liu W, et al. Effects of metformin, acarbose, and sitagliptin monotherapy on gut microbiota in Zucker diabetic fatty rats. BMJ Open Diabetes Res Care. 2019;7(1):e000717.
Gu Y, Wang X, Li J, Zhang Y, Zhong H, Liu R, et al. Analyses of gut microbiota and plasma bile acids enable stratification of patients for antidiabetic treatment. Nat Commun. 2017;8(1):1785.
Baxter NT, et al., The glucoamylase inhibitor acarbose has a diet-dependent and reversible effect on the murine gut microbiome. mSphere, 2019. 4(1).
Smith BJ, Miller RA, Ericsson AC, Harrison DC, Strong R, Schmidt TM. Changes in the gut microbiome and fermentation products concurrent with enhanced longevity in acarbose-treated mice. BMC Microbiol. 2019;19(1):130.
Goeke K, Drepper A, Pape H. Formation of acarbose phosphate by a cell-free extract from the acarbose producer Actinoplanes sp. J Antibiot (Tokyo). 1996;49(7):661–3.
Zhang CS, Podeschwa M, Altenbach HJ, Piepersberg W, Wehmeier UF. The acarbose-biosynthetic enzyme AcbO from Actinoplanes sp. SE 50/110 is a 2-epi-5-epi-valiolone-7-phosphate 2-epimerase. FEBS Lett. 2003;540(1-3):47–52.
Ortseifen V, Kalinowski J, Pühler A, Rückert C. The complete genome sequence of the actinobacterium Streptomyces glaucescens GLA. O (DSM 40922) carrying gene clusters for the biosynthesis of tetracenomycin C, 5’-hydroxy streptomycin, and acarbose. J Biotechnol. 2017;262:84–8.
Tan K, Tesar C, Wilton R, Jedrzejczak RP, Joachimiak A. Interaction of antidiabetic alpha-glucosidase inhibitors and gut bacteria alpha-glucosidase. Protein Sci. 2018;27(8):1498–508.
Weaver GA, Tangel CT, Krause JA, Parfitt MM, Jenkins PL, Rader JM, et al. Acarbose enhances human colonic butyrate production. J Nutr. 1997;127(5):717–23.
Kamimura H, Ogata H, Takahara H. Alpha-glucoside formation of xenobiotics by rat liver alpha-glucosidases. Drug Metab Dispos. 1992;20(2):309–15.
Wolin MJ, Miller TL, Yerry S, Zhang Y, Bank S, Weaver GA. Changes of fermentation pathways of fecal microbial communities associated with a drug treatment that increases dietary starch in the human colon. Appl Environ Microbiol. 1999;65(7):2807–12.
Yang HY, Liu SL, Ibrahim SA, Zhao L, Jiang JL, Sun WF, et al. Oral administration of live Bifidobacterium substrains isolated from healthy centenarians enhanced immune function in BALB/c mice. Nutr Res. 2009;29(4):281–9.
Komura T, Ikeda T, Yasui C, Saeki S, Nishikawa Y. Mechanism underlying prolongevity induced by bifidobacteria in Caenorhabditis elegans. Biogerontology. 2013;14(1):73–87.
Arboleya S, et al. Gut bifidobacteria populations in human health and aging. Front Microbiol. 2016;7:1204.
Yang B, et al. Diversity of gut microbiota and bifidobacterial community of chinese subjects of different ages and from different regions. Microorganisms. 2020:8(8).
Masoro EJ, Compton C, Yu BP, Bertrand H. Temporal and compositional dietary restrictions modulate age-related changes in serum lipids. J Nutr. 1983;113(4):880–92.
Mahoney LB, Denny CA, Seyfried TN. Caloric restriction in C57BL/6J mice mimics therapeutic fasting in humans. Lipids Health Dis. 2006;5:13.
Zhang Y, Xie Y, Berglund ED, Coate KC, He TT, Katafuchi T, et al. The starvation hormone, fibroblast growth factor-21, extends lifespan in mice. Elife. 2012;1:e00065.
Edwards C, et al. D-beta-hydroxybutyrate extends lifespan in C. elegans. Aging (Albany NY). 2014;6(8):621–44.
Veech RL, Bradshaw PC, Clarke K, Curtis W, Pawlosky R, King MT. Ketone bodies mimic the life span extending properties of caloric restriction. IUBMB Life. 2017;69(5):305–14.
Newman JC, Covarrubias AJ, Zhao M, Yu X, Gut P, Ng CP, et al. Ketogenic diet reduces midlife mortality and improves memory in aging mice. Cell Metab. 2017;26(3):547–57 e8.
Roberts MN, Wallace MA, Tomilov AA, Zhou Z, Marcotte GR, Tran D, et al. A ketogenic diet extends longevity and healthspan in adult mice. Cell Metab. 2017;26(3):539–46 e5.
Davis RAH, Deemer SE, Bergeron JM, Little JT, Warren JL, Fisher G, et al. Dietary R, S-1,3-butanediol diacetoacetate reduces body weight and adiposity in obese mice fed a high-fat diet. FASEB J. 2019;33(2):2409–21.
Deemer SE, Davis RAH, Roberts BM, Smith DL Jr, Koutnik AP, Poff AM, et al. Exogenous dietary ketone ester decreases body weight and adiposity in mice housed at thermoneutrality. Obesity (Silver Spring). 2020;28(8):1447–55.
Tseng YH, Tsan YT, Chan WC, Sheu WHH, Chen PC. Use of an alpha-glucosidase inhibitor and the risk of colorectal cancer in patients with diabetes: a nationwide, population-based cohort study. Diabetes Care. 2015;38(11):2068–74.
Liu YC, Nguyen PA, Humayun A, Chien SC, Yang HC, Asdary RN, et al. Does long-term use of antidiabetic drugs changes cancer risk? Medicine (Baltimore). 2019;98(40):e17461.
Dodds SG, Parihar M, Javors M, Nie J, Musi N, Dave Sharp Z, et al. Acarbose improved survival for Apc(+/Min) mice. Aging Cell. 2020;19(2):e13088.
Anisimov VN. Metformin for cancer and aging prevention: is it a time to make the long story short? Oncotarget. 2015;6(37):39398–407.
Anisimov VN. Metformin: do we finally have an anti-aging drug? Cell Cycle. 2013;12(22):3483–9.
Chen YC, Kok VC, Chien CH, Horng JT, Tsai JJ. Cancer risk in patients aged 30 years and above with type 2 diabetes receiving antidiabetic monotherapy: a cohort study using metformin as the comparator. Ther Clin Risk Manag. 2015;11:1315–23.
Anisimov VN. Metformin for prevention and treatment of colon cancer: a reappraisal of experimental and clinical data. Curr Drug Targets. 2016;17(4):439–46.
Zhao B, Luo J, Yu T, Zhou L, Lv H, Shang P. Anticancer mechanisms of metformin: a review of the current evidence. Life Sci. 2020;254:117717.
Rena G, Hardie DG, Pearson ER. The mechanisms of action of metformin. Diabetologia. 2017;60(9):1577–85.
Sahdeo S, Tomilov A, Komachi K, Iwahashi C, Datta S, Hughes O, et al. High-throughput screening of FDA-approved drugs using oxygen biosensor plates reveals secondary mitofunctional effects. Mitochondrion. 2014;17:116–25.
Chiasson, J.L., Acarbose for the prevention of diabetes, hypertension, and cardiovascular disease in subjects with impaired glucose tolerance: the Study to Prevent Non-Insulin-Dependent Diabetes Mellitus (STOP-NIDDM) Trial. Endocr Pract, 2006. 12 Suppl 1: p. 25-30.
DeFronzo RA, bdul-Ghani MA. Preservation of beta-cell function: the key to diabetes prevention. J Clin Endocrinol Metab. 2011;96(8):2354–66.
Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M, et al. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trial. JAMA. 2003;290(4):486–94.
Delorme S, Chiasson JL. Acarbose in the prevention of cardiovascular disease in subjects with impaired glucose tolerance and type 2 diabetes mellitus. Curr Opin Pharmacol. 2005;5(2):184–9.
Moelands SV, et al. Alpha-glucosidase inhibitors for prevention or delay of type 2 diabetes mellitus and its associated complications in people at increased risk of developing type 2 diabetes mellitus. Cochrane Database Syst Rev. 2018;12:CD005061.
Hsu PF, Sung SH, Cheng HM, Shin SJ, Lin KD, Chong K, et al. Cardiovascular benefits of acarbose vs sulfonylureas in patients with type 2 diabetes treated with metformin. J Clin Endocrinol Metab. 2018;103(10):3611–9.
Ogawa S, Takeuchi K, Ito S. Acarbose lowers serum triglyceride and postprandial chylomicron levels in type 2 diabetes. Diabetes Obes Metab. 2004;6(5):384–90.
Zheng MY, Yang JH, Shan CY, Zhou HT, Xu YG, Wang Y, et al. Effects of 24-week treatment with acarbose on glucagon-like peptide 1 in newly diagnosed type 2 diabetic patients: a preliminary report. Cardiovasc Diabetol. 2013;12:73.
McCarty, M.F. and J.J. DiNicolantonio, Acarbose, lente carbohydrate, and prebiotics promote metabolic health and longevity by stimulating intestinal production of GLP-1. Open Heart, 2015. 2(1): p. e000205.
Frantz S, Calvillo L, Tillmanns J, Elbing I, Dienesch C, Bischoff H, et al. Repetitive postprandial hyperglycemia increases cardiac ischemia/reperfusion injury: prevention by the alpha-glucosidase inhibitor acarbose. FASEB J. 2005;19(6):591–3.
Cardoso S, Moreira PI. Antidiabetic drugs for Alzheimer’s and Parkinson’s diseases: repurposing insulin, metformin, and thiazolidinediones. Int Rev Neurobiol. 2020;155:37–64.
Luchsinger JA. Type 2 diabetes, related conditions, in relation and dementia: an opportunity for prevention? J Alzheimers Dis. 2010;20(3):723–36.
Tseng CH. Dementia risk in type 2 diabetes patients: acarbose use and its joint effects with metformin and pioglitazone. Aging Dis. 2020;11(3):658–67.
Chen HH, et al. Acarbose decreases the rheumatoid arthritis risk of diabetic patients and attenuates the incidence and severity of collagen-induced arthritis in mice. Sci Rep. 2015;5:18288.
Zhang L, et al. alpha-Glucosidase inhibitors alter gut microbiota and ameliorate collagen-induced arthritis. Front Pharmacol. 2019;10:1684.
Derosa G, Maffioli P, Ferrari I, Fogari E, D'Angelo A, Palumbo I, et al. Acarbose actions on insulin resistance and inflammatory parameters during an oral fat load. Eur J Pharmacol. 2011;651(1-3):240–50.
Mo D, Liu S, Ma H, Tian H, Yu H, Zhang X, et al. Effects of acarbose and metformin on the inflammatory state in newly diagnosed type 2 diabetes patients: a one-year randomized clinical study. Drug Des Devel Ther. 2019;13:2769–76.
Lin YC, Chen YC, Hsiao HP, Kuo CH, Chen BH, Chen YT, et al. The effects of acarbose on chemokine and cytokine production in human monocytic THP-1 cells. Hormones (Athens). 2019;18(2):179–87.
Rios JL, Francini F, Schinella GR. Natural products for the treatment of type 2 diabetes mellitus. Planta Med. 2015;81(12-13):975–94.
Davis PA, Yokoyama W. Cinnamon intake lowers fasting blood glucose: meta-analysis. J Med Food. 2011;14(9):884–9.
Pereira ASP, et al. Evaluation of the anti-diabetic activity of some common herbs and spices: providing new insights with inverse virtual screening. Molecules. 2019:24(22).
Medagama AB. The glycaemic outcomes of Cinnamon, a review of the experimental evidence and clinical trials. Nutr J. 2015;14:108.
Hosoda K, Wang MF, Liao ML, Chuang CK, Iha M, Clevidence B, et al. Antihyperglycemic effect of oolong tea in type 2 diabetes. Diabetes Care. 2003;26(6):1714–8.
Tang GY, et al. Health functions and related molecular mechanisms of tea components: an update review. Int J Mol Sci. 2019:20(24).
Adisakwattana S, Lerdsuwankij O, Poputtachai U, Minipun A, Suparpprom C. Inhibitory activity of cinnamon bark species and their combination effect with acarbose against intestinal alpha-glucosidase and pancreatic alpha-amylase. Plant Foods Hum Nutr. 2011;66(2):143–8.
Gao J, Xu P, Wang Y, Wang Y, Hochstetter D. Combined effects of green tea extracts, green tea polyphenols or epigallocatechin gallate with acarbose on inhibition against alpha-amylase and alpha-glucosidase in vitro. Molecules. 2013;18(9):11614–23.
Garratt M, Bower B, Garcia GG, Miller RA. Sex differences in lifespan extension with acarbose and 17-alpha estradiol: gonadal hormones underlie male-specific improvements in glucose tolerance and mTORC2 signaling. Aging Cell. 2017;16(6):1256–66.
Smith DL Jr, Nagy TR, Allison DB. Calorie restriction: what recent results suggest for the future of ageing research. Eur J Clin Investig. 2010;40(5):440–50.
Miller RA, Harrison DE, Astle CM, Fernandez E, Flurkey K, Han M, et al. Rapamycin-mediated lifespan increase in mice is dose and sex dependent and metabolically distinct from dietary restriction. Aging Cell. 2014;13(3):468–77.
List JF, Whaley JM. Glucose dynamics and mechanistic implications of SGLT2 inhibitors in animals and humans. Kidney Int Suppl. 2011;120:S20–7.
Shintani H, et al. Calorie restriction mimetics: upstream-type compounds for modulating glucose metabolism. Nutrients. 2018:10(12).
Zhang W, Welihinda A, Mechanic J, Ding H, Zhu L, Lu Y, et al. EGT1442, a potent and selective SGLT2 inhibitor, attenuates blood glucose and HbA(1c) levels in db/db mice and prolongs the survival of stroke-prone rats. Pharmacol Res. 2011;63(4):284–93.
Funding
Funding in support of this work is derived in part by NIH grant award P30AG050886.
Author information
Authors and Affiliations
Contributions
All authors provided input and final approval of the submitted and published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Daniel L. Smith Jr has no conflicts relevant to the content of the review article. Dr. Smith’s institution, the University of Alabama at Birmingham, has received funds to support research from the National Institutes of Health, McCormick Science Institute and the Alliance for Potato Research and Education. Rachael M. Orlandella has no conflicts relevant to the content of this review article. Lyse A. Norian has no conflicts relevant to the content of the review article. In the last thirty-six months, Dr. Allison has received personal payments or promises for the same from the following: American Society for Nutrition; Alkermes, Inc.; American Statistical Association; Biofortis; California Walnut Commission; Columbia University; Fish & Richardson, P.C.; Frontiers Publishing; Henry Stewart Talks; IKEA; Indiana University; Laura and John Arnold Foundation; Johns Hopkins University; Law Offices of Ronald Marron; MD Anderson Cancer Center; Medical College of Wisconsin; National Institutes of Health (NIH); Sage Publishing; The Obesity Society; Soleno Therapeutics; Tomasik, Kotin & Kasserman LLC; University of Alabama at Birmingham; University of Miami; Nestle; WW (formerly Weight Watchers International, LLC). Donations to a foundation have been made on his behalf by the Northarvest Bean Growers Association. Dr. Allison has been an unpaid member of the International Life Sciences Institute North America Board of Trustees. Dr. Allison’s institution, Indiana University, has received funds to support his research or educational activities from the following: NIH; Eli Lilly, Alliance for Potato Research and Education; American Federation for Aging Research; Dairy Management Inc.; Herbalife; Laura and John Arnold Foundation; National Cattlemen’s Beef Association, Oxford University Press, the Sloan Foundation, The Gordan and Betty Moore Foundation, and numerous other for-profit and non-profit organizations to support the work of the School of Public Health and the university more broadly.
Disclaimer
The opinions expressed are those of the authors and do not necessarily represent those of the NIH or any other organization with which the authors are affiliated.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Smith, D.L., Orlandella, R.M., Allison, D.B. et al. Diabetes medications as potential calorie restriction mimetics—a focus on the alpha-glucosidase inhibitor acarbose. GeroScience 43, 1123–1133 (2021). https://doi.org/10.1007/s11357-020-00278-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-020-00278-x