Abstract
Neurexin1 (Nrxn1) and Neuroligin3 (Nlgn3) are cell adhesion proteins, which play an important role in synaptic plasticity that declines with advancing age. However, the expression of these proteins during aging has not been analyzed. In the present study, we have examined the age-related changes in the expression of these proteins in cerebral cortex and hippocampus of 10-, 30-, 50-, and 80-week-old male mice. Reverse transcriptase polymerase chain reaction (RT-PCR) analysis indicated that messenger RNA (mRNA) level of Nrxn1 and Nlgn3 significantly increased from 10 to 30 weeks and then decreased at 50 weeks in both the regions. However, in 80-week-old mice, Nrxn1 and Nlgn3 were further downregulated in cerebral cortex while Nrxn1 was downregulated and Nlgn3 was upregulated in hippocampus. These findings were corroborated by immunoblotting and immunofluorescence results. When the expression of Nrxn1 and Nlgn3 was correlated with presynaptic density marker synaptophysin, it was found that synaptophysin protein expression in cerebral cortex was high at 10 weeks and decreased gradually up to 80 weeks, whereas in hippocampus, it decreased until 50 weeks and then increased remarkably at 80 weeks. Furthermore, Pearson’s correlation analysis showed that synaptophysin had a strong relation with Nrxn1 and Nlgn3 in cerebral cortex and with Nlgn3 in hippocampus. Thus, these findings showed that Nrxn1 and Nlgn3 are differentially expressed in cerebral cortex and hippocampus which might be responsible for alterations in synaptic plasticity during aging.




Similar content being viewed by others
Change history
04 September 2019
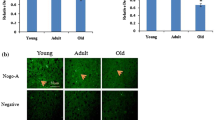
The corresponding author noticed in his published paper that the images (30 weeks, CC, 10, 30 and 50 weeks DG) of fig. 3b are inadvertently duplicated with the images of fig. 3a. Now, these images have been replaced in the corrected panel (Fig. 3b) below.
04 September 2019
The corresponding author noticed in his published paper that the images (30 weeks, CC, 10, 30 and 50 weeks DG) of fig. 3b are inadvertently duplicated with the images of fig. 3a. Now, these images have been replaced in the corrected panel (Fig. 3b) below.
References
Arac D, Boucard AA, Ozkan E, Strop P, Newell E, Südhof TC, Brunger AT (2007) Structures of neuroligin-1 and the neuroligin-1/neurexin-1 beta complex reveal specific protein-protein and protein-Ca2+ interactions. Neuron 56:992–1003
Benice TS, Rizk A, Kohama S, Pfankuch T, Raber J (2006) Sex-differences in age-related cognitive decline in C57BL/6 J mice associated with increased brain microtubule-associated protein 2 and synaptophysin immunoreactivity. Neuroscience 137:413–423
Bloss EB, Janssen WG, Ohm DT, Yuk FJ, Wadsworth S, Saardi KM, McEwen BS, Morrison JH (2011) Evidence for reduced experience-dependent dendritic spine plasticity in the aging prefrontal cortex. J Neurosci 31:7831–7839
Bloss EB, Puri R, Yuk F, Punsoni M, Hara Y, Janssen WG, McEwen BS, Morrison JH (2013) Morphological and molecular changes in aging rat prelimbic prefrontal cortical synapses. Neurobiol Aging 34:200–210
Bradford MM (1976) Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Budreck EC, Scheiffele P (2007) Neuroligin-3 is a neuronal adhesion protein at GABAergic and glutamatergic synapses. Eur J Neurosci 26:1738–1748
Burke SN, Barnes CA (2006) Neural plasticity in the ageing brain. Nat Rev Neurosci 7:30–40
Chen X, Liu H, Shim AHR, Focia PJ, He X (2008) Structural basis for synaptic adhesion mediated by neuroligin–neurexin interactions. Nat Struct Mol Biol 15:50–56
Chubykin AA, Atasoy D, Etherton MR, Brose N, Kavalali ET, Gibson JR, Sudhof TC (2007) Activity-dependent validation of excitatory versus inhibitory synapses by neuroligin-1 versus neuroligin-2. Neuron 54:919–931
Comoletti D, De Jaco A, Jennings LL, Flynn RE, Gaietta G, Tsigelny I, Ellisman MH, Taylor P (2004) The Arg451Cys neuroligin-3 mutation associated with autism reveals a defect in protein processing. J Neurosci 24:4889–4893
Dean C, Dresbach T (2006) Neuroligins and neurexins: linking cell adhesion, synapse formation and cognitive function. Trends Neurosci 29:21–29
Dean C, Scholl FG, Choih J, DeMaria S, Berger J, Isacoff E, Scheiffele P (2003) Neurexin mediates the assembly of presynaptic terminals. Nat Neurosci 6:708–716
Driscoll I, Howard SR, Stone JC, Monfils MH, Tomanek B, Brooks WM, Sutherland RJ (2006) The aging hippocampus: a multi-level analysis in the rat. Neuroscience 139:1173–1185
Dumitriu D, Hao JD, Hara Y, Kaufmann J, Janssen WGM, Lou W, Rapp PR, Morrison JH (2010) Selective changes in thin spine density and morphology in monkey prefrontal cortex correlate with age-related cognitive impairment. J Neurosci 30:7507–7515
El-Husseini A, Schnell E, Chetkovich DM, Nicoll RA, Bredt DS (2000) PSD95 involvement in maturation of excitatory synapses. Science 290:1364–1368
Frick KM, Fernandez SM (2003) Enrichment enhances spatial memory and increases synaptophysin levels in aged female mice. Neurobiol Aging 24:615–626
Geinisman Y, Detoledo-Morrell L, Morrell F, Heller RE (1995) Hippocampal markers of age-related memory dysfunction: behavioral, electrophysiological and morphological perspectives. Prog Neurobiol 45:223–252
Gleichmann M, Zhang Y, Wood WH III, Becker KG, Mughal MR, Pazin MJ, Praaga H, Kobiloa T, Zonderman AB, Troncoso JC, Markesbery WR, Mattson MP (2012) Molecular changes in brain aging and Alzheimer’s disease are mirrored in experimentally silenced cortical neuron networks. Neurobiol Aging 33:205e1–205e18
Graf ER, Zhang X, Jin SX, Linhoff MW, Craig AM (2004) Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins. Cell 119:1013–1026
Gwendolen EH, Steven GK, Henryk FU, Jacob R (2010) Age-related decreases in SYN levels associated with increases in MAP-2, apoE, and GFAP levels in the rhesus macaque prefrontal cortex and hippocampus. Age 32:283–296
Hara Y, Rapp PR, Morrison JH (2012) Neuronal and morphological base of cognitive decline in aged rhesus monkeys. Age 34:1051–1073
King DL, Arendash GW (2002) Maintained synaptophysin immunoreactivity in Tg2576 transgenic mice during aging: correlations with cognitive impairment. Brain Res 926:58–68
Kolozsi E, Mackenzie RN, Roullet FI, deCatanzaro D, Foster JA (2009) Prenatal exposure to valproic acid leads to reduced expression of synaptic adhesion molecule neuroligin 3 in mice. Neuroscience 163:1201–1210
Krueger DD, Tuffy LP, Papadopoulos T, Brose N (2012) The role of neurexins and neuroligins in the formation, maturation, and function of vertebrate synapses. Curr Opin Neurobiol 22:412–422
Kumar D, Thakur MK (2014) Perinatal exposure to bisphenol-A impairs spatial memory through upregulation of neurexin1 and neuroligin3 expression in male mouse brain. PLoS ONE 9:e110482
Labarrière M, Thomas F, Dutar P, Pollegioni L, Wolosker H, Billard JM (2014) Circuit-specific changes in D-serine-dependent activation of the N-methyl-D-aspartate receptor in the aging hippocampus. Age 36:9698
Levy R, Goldman-Rakic P (1999) Association of storage and processing functions in the dorsolateral prefrontal cortex of the non-human primate. J Neurosci 19:5149–5158
Maguire EA, Cipolotti L (1998) Selective sparing of topographical memory. J Neurol Neurosurg Psychiatry 65:903–909
Martin SJ, Grimwood PD, Morris RG (2000) Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci 23:649–711
Michael JF (2006) Rodent models of brain aging and neurodegeneration. Age 28:219–220
Morrison JH, Baxter MG (2012) The ageing cortical synapse: hallmarks and implications for cognitive decline. Nat Rev Neurosci 13:240–250
Mulder M, Jansen PJ, Janssen BJ, van de Berg WD, van der Boom H, Havekes LM, de Kloet RE, Ramaekers FC, Blokland A (2004) Low-density lipoprotein receptor-knockout mice display impaired spatial memory associated with a decreased synaptic density in the hippocampus. Neurobiol Dis 16:212–219
Nguyen T, Sudhof TC (1997) Binding properties of neuroligin 1 and neurexin 1beta reveal function as heterophilic cell adhesion molecules. J Biol Chem 272:26032–26039
Paulesu E, Frith C, Frackowiak R (1993) The neural correlates of the verbal component of working memory. Nature 362:342–345
Puschel AW, Betz H (1995) Neurexins are differentially expressed in the embryonic nervous system of mice. J Neurosci 15:2849–2856
Rapp PR, Kansky MT, Roberts JA (1997) Impaired spatial information processing in aged monkeys with preserved recognition memory. NeuroReport 8:1923–1928
Reddy PH, Mani G, Park BS, Jacques J, Murdoch G, Whetsell W Jr, Kaye J, Manczak M (2005) Differential loss of synaptic proteins in Alzheimer’s disease: implications for synaptic dysfunction. J Alzheimers Dis 7:103–117
Rosenbaum RS, Winocur G, Moscovitch M (2001) New views on old memories: re-evaluating the role of the hippocampal complex. Behav Brain Res 127:183–97.
Scheiffele P, Fan J, Choih J, Fetter R, Serafini T (2000) Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell 101:657–669
Sibille E (2013) Molecular aging of the brain, neuroplasticity and vulnerability to depression and other brain-related disorders. Dialogues Clin Neurosci 15:53–56
Sohal RS, Brunk UT (1989) Lipofuscin as an indicator of oxidative stress and aging. Adv Exp Med Biol 266:17–26
Song JY, Ichtchenko K, Südhof TC, Brose N (1999) Neuroligin 1 is a postsynaptic cell-adhesion molecule of excitatory synapses. Proc Natl Acad Sci U S A 96:1100–1105
Südhof TC (2008) Neuroligins and neurexins link synaptic function to cognitive disease. Nature 455:903–911
Tarsa L, Goda Y (2002) Synaptophysin regulates activity-dependent synapse formation in cultured hippocampal neurons. Proc Natl Acad Sci U S A 99:1012–1016
Tisserand DJ, Jolles J (2003) On the involvement of prefrontal networks in cognitive ageing. Cortex 39:1107–1128
Ushkaryov YA, Petrenko AG, Geppert M, Sudhof TC (1992) Neurexins: synaptic cell surface proteins related to the alpha-latrotoxin receptor and laminin. Science 257:50–56
Van der Jeugd A, Vermaercke B, Derisbourg M, Lo AC, Hamdane M, Blum D, Buée L, D'Hooge R (2013) Progressive age-related cognitive decline in tau mice. J Alzheimers Dis 37:777–788
Winocur G, Gagnon S (1998) Glucose treatment attenuates spatial learning and memory deficits of aged rats on tests of hippocampal function. Neurobiol Aging 19:233–241
Wittenmayer N, Körber C, Liu H, Kremer T, Varoqueaux F, Chapman ER, Brose N, Kuner T, Dresbach T (2009) Postsynaptic neuroligin1 regulates presynaptic maturation. PNAS 106:13564–13569
Xu XH, Ouyang J, Xie PH, Chen JH (2008) Changes of activity and expression of protein phosphatase type 2A during the apoptosis of NB4 and MR2 cells induced by arsenic trioxide. Zhongguo Shi Yan Xue Ye Xue Za Zhi 16:1021–1025
Yamagata M, Sanes JR, Weiner JA (2003) Synaptic adhesion molecules. Curr Opin Cell Biol 155:621–632
Yang L, Zhang J, Zheng K, Shen H, Chen X (2014) Long-term ginsenoside Rg1 supplementation improves age-related cognitive decline by promoting synaptic plasticity associated protein expression in C57BL/6 J mice. J Gerontol A Biol Sci Med Sci 69:282–294
Zhang C, Milunsky JM, Newton S, Ko J, Zhao G, Maher TA, Tager-Flusberg H, Bolliger MF, Carter AS, Boucard AA, Powell CM, Südhof TC (2009) A neuroligin-4 missense mutation associated with autism impairs neuroligin-4 folding and endoplasmic reticulum export. J Neurosci 29:10843–10854
Zhao C, Deng W, Gage FH (2008) Mechanisms and functional implications of adult neurogenesis. Cell 132:645–660
Acknowledgments
The present study was supported by grants from the Department of Biotechnology, Government of India, to MKT [BT/PR3996/MED/97/57/2011], and research fellowship to DK from the Council of Scientific Industrial Research, India [09/013(0253)/2009-EMR-I].
Conflict of interest
The authors do not have any potential conflict of interest including any financial, personal, or academic aspect.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Kumar, D., Thakur, M.K. Age-related expression of Neurexin1 and Neuroligin3 is correlated with presynaptic density in the cerebral cortex and hippocampus of male mice. AGE 37, 17 (2015). https://doi.org/10.1007/s11357-015-9752-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11357-015-9752-6